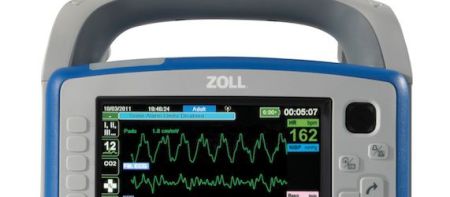
ZOLL Medical Corporation, a manufacturer of medical devices and related software solutions, announced today that its Japanese subsidiary, Asahi Kasei ZOLL Medical (AZM), has received Shonin approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) to enter the Japanese market with its X Series® Monitor/Defibrillator.
AZM, a ZOLL company established in August 2012 to pursue the regulatory approval, sale, and marketing of ZOLL products in Japan, will begin the marketing and selling of the X Series in spring 2014. Designed primarily for EMS operations and critical care transport, the X Series is about half the size and half the weight of competitive full- featured monitor/defibrillators, yet much more powerful and built to the most extensive standards for ruggedness.
Weighing less than 12 pounds (6 kilograms), the X Series is compact without compromise in capability, performance, or display size. This monitor/defibrillator combines the clinically superior therapeutic capabilities of ZOLL defibrillation, pacing, and CPR assistance with advanced monitoring parameters. Key among the X Series’ features is the unequaled ability to assist with improving CPR quality to keep oxygen-rich blood flowing. ZOLL’s proprietary See-Thru CPR® minimizes the duration of pauses in CPR to enable rescuers to see the underlying rhythm. ZOLL’s Real CPR Help® provides real-time CPR feedback, and a built-in sensor measures the depth, rate, and effectiveness of chest compressions.
The X Series also lets first responders assess the patient’s needs as soon as possible and wirelessly transmit critical information to a receiving hospital so clinicians are prepared when the ambulance arrives. Wireless transmission allows the EMS crew and hospital staff to work as a team and start treating the patient without delay.
“We are very excited to begin commercialization of the X Series in Japan and to continue to offer innovative technologies and devices for acute critical care to this high-growth market,” said Richard A. Packer, CEO of ZOLL. “The X Series not only helps rescuers save lives, but makes the lives of first responders a great deal easier by having so many life-saving capabilities in one unit.”
In addition, the X Series provides ECG monitoring, invasive and non-invasive blood pressure, pulse oximetry (including SpO2, CO, etc.), end-tidal carbon dioxide concentration (EtCO2), body temperature, and respiratory rate.
Other ZOLL products that received Shonin approval in Japan:
- The AED Plus® is an automated external defibrillator (AED) that incorporates ZOLL’s real-time CPR feedback technology, Real CPR Help®. The AED Plus is the only AED with CPR feedback capability available in the Japanese market.
- The AutoPulse® Non-Invasive Cardiac Support Pump is an automated, portable chest compression device. It is the only mechanical CPR system to have shown improved survival in comparative clinical trials. TheAutoPulse more than tripled survival compared to typical CPR during witnessed shockable arrests.1
- ZOLL’s Intravascular Temperature Management (IVTMTM) system offers health care providers the power and control they need to rapidly, safely, and effectively manage the core body temperature of critically ill or surgical patients. In Japan, IVTM is only approved for patients with high fever. The Thermogard XP® delivers accurate, easy-to-use and cost-effective control for both cooling and warming applications.
Source: Zoll
16 December 2013
Latest Articles
CPR, Cardiac, ZOLL, cardiac arrest, defibrillator, AED
ZOLL Medical Corporation, a manufacturer of medical devices and related software solutions, announced today that its Japanese subsidiary, Asahi Kasei ZOLL...