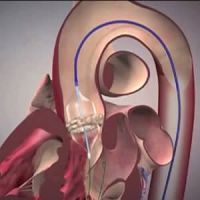
In the Placement of Aortic Transcatheter Valves (PARTNER) 3 trial, 1,000 patients with severe aortic stenosis and low surgical risk were randomly assigned to undergo either transcatheter aortic-valve replacement (TAVR) or surgical aortic-valve replacement. In these patients, TAVR with transfemoral placement of a balloon-expandable valve was superior to surgery with regard to the primary composite end point of death, stroke, or rehospitalisation at 1 year, according to the findings published in The New England Journal of Medicine.
Previous randomised trials of TAVR with both balloon-expandable valves and self-expanding valves showed that, in patients who were at intermediate or high risk for death with surgery, TAVR was either superior or noninferior to standard therapies, including surgical aortic-valve replacement.
Moreover, technological enhancements and procedural simplification have contributed to increased use of TAVR, such that more patients now undergo TAVR than isolated surgery for aortic-valve replacement in the United States. However, most patients with severe aortic stenosis are at low surgical risk, and there is insufficient evidence regarding the comparison of TAVR with surgery in such patients.
The PARTNER 3 trial was conducted at 71 centres where 1,000 low-risk patients underwent randomisation. The mean age of the patients was 73 years, and the mean Society of Thoracic Surgeons risk score was 1.9% (with scores ranging from 0 to 100% and higher scores indicating a greater risk of death within 30 days after the procedure).
Key findings of the trial include:
- The rate of the primary composite end point at 1 year was significantly lower in the TAVR group than in the surgery group (8.5% vs. 15.1%; absolute difference, −6.6 percentage points; 95% confidence interval [CI], −10.8 to −2.5; P<0.001 for noninferiority; hazard ratio, 0.54; 95% CI, 0.37 to 0.79; P=0.001 for superiority).
- At 30 days, TAVR resulted in a lower rate of stroke than surgery (P=0.02) and in lower rates of death or stroke (P=0.01) and new-onset atrial fibrillation (P<0.001).
- TAVR also resulted in a shorter index hospitalisation than surgery (P<0.001) and in a lower risk of a poor treatment outcome (death or a low Kansas City Cardiomyopathy Questionnaire score) at 30 days (P<0.001).
- There were no significant between-group differences in major vascular complications, new permanent pacemaker insertions, or moderate or severe paravalvular regurgitation.
Current clinical practice has restricted the use of TAVR in patients who are at low risk and in younger patients, for whom surgery is standard therapy. Previous research that supports the use of TAVR in low-risk patients is limited, mostly consisting of retrospective, observational studies.
The most important limitation of the PARTNER 3 trial is that current results reflect only 1-year outcomes and do not address the problem of long-term structural valve deterioration. Definitive conclusions regarding the advantages and disadvantages of TAVR as compared with surgery (with either bioprosthetic or mechanical valves) depend on long-term follow-up. In this trial involving younger, low-risk patients, the protocol requires clinical and echocardiographic follow-up to continue for at least 10 years.
Another limitation is that in this trial, as in previous TAVR trials, adjudication of end points was not blinded, which could have resulted in bias in outcome assessment.
Source: The New England Journal of Medicine
Image credit: Pixabay