ICU Management & Practice, Volume 16 - Issue 4, 2016
ARDS is Heterogeneous
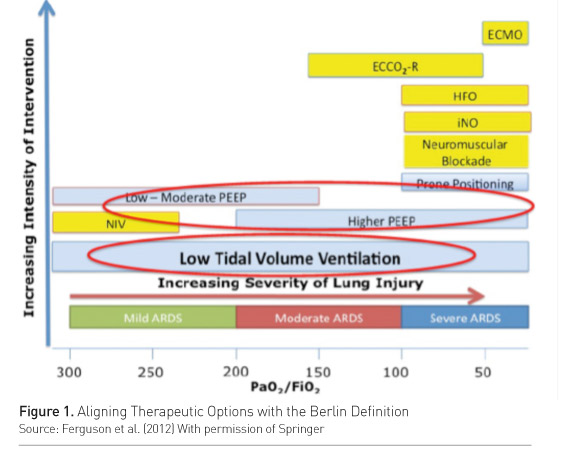
Acute respiratory distress syndrome (ARDS) is a heterogeneous entity. Calfee and colleagues’ analysis of the ARMA and ALVEOLI trials (Calfee et al. 2015) differentiated two ARDS subphenotypes, one of which was categorized by more severe inflammation and worse clinical outcomes. Response to positive end-expiratory pressure (PEEP) was different in the two subphenotypes. High PEEP showed more ventilator-free days and organ failure-free days and increased survival only in the subphenotype characterized by a greater inflammation. ARDS severity also affects the response to treatment. In a meta-analysis on studies of high vs. low PEEP in ARDS, Briel and colleagues showed that higher PEEP reduced the risk of death and shortened the time to unassisted breathing only in moderateto-severe ARDS cases (Briel et al. 2010). In mild ARDS the opposite may be true. So, the same treatment may change the outcome according to the phenotype and to severity. This concept has been incorporated in current recommendations (Ferguson et al. 2012), stating that higher PEEP should be reserved to moderate-to-severe ARDS cases (Figure 1).
 Why and How
to Individualize Ventilation
Why and How
to Individualize Ventilation
Individualized treatment has the potential to improve patient outcome and reduce side effects of treatment in patients who do not respond, thus allowing better use of resources. Protective ventilation is currently used in ARDS and it is based on the application of low tidal volume (Vt) and moderateto-high PEEP with the aim of avoiding overdistension and optimizing recruitment. Individualising protective ventilation in ARDS means selecting the right tidal volume and the right level of PEEP for each individual patient.
ARDS is a restrictive disease and this is well reflected in the concept of the “baby lung” (the size of the aerated lung still accessible to ventilation is reduced to the size of the lung of a baby). The obvious implication is that the size of the “baby” lung should determine the ventilator settings. Decreasing Vt from 12 ml/kg of predicted body weight (PBW) to 6 ml/kg PBW was shown to improve survival, likely because of the decrease in lung overdistention (Acute Respiratory Distress Syndrome Network 2000). A Vt of 6 ml/kg PBW does not assure that lung overdistension is always avoided in every patient. A smaller “baby” lung can in fact be hyperinflated even using a Vt of 6 ml/kg PBW (Terragni et al. 2007), suggesting that Vt should be better tailored on the size of the baby lung than on the body weight (Gattinoni et al. 2016). Modern ventilators have the technology to measure directly the aerated lung volume thus allowing to normalize tidal volume on the size of the baby lung.
Another way to try to normalize the tidal volume to the size of the baby lung is to use the compliance of the respiratory system (Crs). Compliance in ARDS is not low because the lung is stiff, it is low because the aerated lung is small. Thus, compliance is a good index of normally aerated lung tissue and can give an estimation of the baby lung size.
Normalising tidal volume to the compliance of the respiratory system gives the driving pressure (Vt/Crs). The driving pressure (ΔP) can be calculated at the bedside as plateau pressure minus PEEP (Pplat – PEEP), and it can be considered as an estimate of the lung strain. Lung strain is the lung deformation imposed by tidal ventilation, and it is equal to the ratio of tidal volume divided by the functional residual capacity, that is the size of the aerated baby lung at end-expiration.
Lung Strain (ΔP)= Tidal volume (Vt)
Size of the
baby lung (Crs)
An analysis of data on 3562 ARDS patients examined the relationship between driving pressure and survival (Amato et al. 2015), and found that driving pressure was the ventilation variable most strongly associated with survival. Their analysis demonstrated that the mortality rate paralleled changes in tidal volume only when it is expressed as a function of compliance, which is an estimation of the baby lung, that is the driving pressure (Vt/Crs). On the contrary, mortality rate was not correlated with tidal volume when it is expressed as a function of body weight (Vt/PBW). This shows clearly that the tidal volume should not be sized on patient’ predicted body weight but on the size of the baby lung.
There is a relationship between the size of the baby lung, recruitability, recruitment, tidal volume and PEEP. Richard and colleagues demonstrated that low tidal volume promotes alveolar derecruitment that can be prevented by an increase in PEEP (Richard et al. 2003). This study found that, for a given plateau pressure (i.e., similar end-inspiratory distension), a high PEEP-low Vt strategy increased recruitment and PaO2 as compared to a low PEEP-high Vt strategy, suggesting that the effect of PEEP on recruitment is greater than that of Vt. By promoting alveolar recruitment, PEEP may increase the size of the baby lung, allowing a better accommodation of tidal volume, which is reflected by a decrease in the driving pressure. Thus, PEEP is a measure that can increase the size of the baby lung.
High PEEP is not recommended for all ARDS patients. Two trials comparing high vs. low PEEP failed to show any advantages of an indiscriminate use of high PEEP in all ARDS patients (Brower et al. 2004; Meade et al. 2008). In fact, it is logical to use higher PEEP only in patients who have some parts of the lungs that can be recruited. Recruitability (lung that can be recruited) is clearly correlated with recruitment (lung that is actually recruited) (Gattinoni et al. 2006), suggesting that PEEP should only be applied when there is a potential for recruitment. Gattinoni showed that patients with highly recruitable lung have more severe and more diffuse injury, i.e., they have a smaller baby lung at low PEEP (Gattinoni et al. 2006).
Caironi and colleagues (2015) further elucidated this concept. These authors differentiated patients according to the amount of cyclic lung opening (during inflation) and closing (during deflation), which is a measure of recruitability (the higher the cyclic opening-closing, the higher the recruitability). They demonstrated that increasing PEEP decreases the cyclic opening and closing (i.e., increases lung recruitment) only in patients with higher recruitability. In addition, the increased recruitment obtained with PEEP in these patients was correlated with an increased survival. Thus, using high PEEP increases recruitment when there is a high potential for recruitment and this may improve outcome. Increasing PEEP has no effect and may even be detrimental in patients with a lower potential for recruitment.
How to
Assess Recruitment to Set PEEP at the Bedside
PEEP is used to recruit the lung. Measuring or estimating lung recruitment is therefore important for optimizing the PEEP setting.
A simple way to assess recruitment induced by PEEP is to measure the lung volume. Dellamonica and colleagues (2011) compared a method to estimate alveolar recruitment derived from bedside measurement of end-expiratory lung volume (nitrogen washout/washin technique) with the measurement of recruitment obtained on the pressure volume curves (standard technique). Estimated recruitment with increasing PEEP was expressed by: ΔPEEP (ΔEELV x Crs at low PEEP). They showed that the two methods yielded similar results, thus demonstrating that the nitrogen washout/washin technique can be used for bedside assessment of PEEP-induced recruitment.
Changes in oxygenation can be used to estimate recruitment. Maggiore and coworkers reported that a significant correlation exists between recruitment and oxygenation (Maggiore et al. 2001). This correlation, however, is too weak to allow, in an individual patient, to assess PEEP-induced recruitment by its effect on oxygenation.
Maggiore and colleagues also found a very tight correlation between compliance at zero or low PEEP and recruitment obtained with PEEP 15 cm H2O (Maggiore 2001). In other words, compliance is an estimate of recruitability—the higher the compliance, the higher the recruitment with PEEP.
It is possible to use compliance to individualize the PEEP setting according to recruitability. Let’s imagine to ventilate an ARDS patient with a Vt 6mL/kg PBW and to keep the plateau pressure at the safe limit of 28-30 cmH2O. If the compliance at low PEEP (e.g., 5 cm H2O) is high, the pressure oscillation due to tidal ventilation (i.e., the driving pressure) will be small. So the maximum PEEP that can be applied to reach a plateau pressure of 28-30 cm H2O will be high. The opposite will occur if the compliance at low PEEP is low. In this case, tidal volume will produce a higher driving pressure, so that the maximum PEEP that you can use to reach the target plateau pressure is low, because much of the pressure is already taken by the driving pressure. This is a way to individualize the PEEP setting in order to maximize recruitment in a safe way and it was used in the EXPRESS trial.
The EXPRESS trial compared a moderate PEEP strategy (5-9 cm H2O), to minimize overdistension, to a PEEP setting to safely maximize recruitment, as described above. In this study, there was no difference in mortality, but there were more patients breathing without ventilator assistance when PEEP was individually set to maximize recruitment. In more severe patients, there was a clear trend to a lower mortality and a significantly higher number of patients breathing without ventilator assistance when PEEP was set to maximize recruitment. On the contrary, higher PEEP had no effect in patients with mild ARDS (Mercat et al. 2008). We also need to consider cases where mechanical ventilation fails, i.e., patients with a too small baby lung to allow for a safe conventional mechanical ventilation. In these patients, Terragni and colleagues (2009) showed that use of extracorporeal carbon dioxide removal (ECCO2R) allowed to provide ultraprotective ventilation with a reduction of tidal volume below 6 ml/kg PBW (up to 4 ml/kg).
Conclusion
The future of mechanical ventilation in ARDS is an individualized approach. First of all, intensivists need to become better at recognising ARDS. As shown by the LUNG-SAFE study (Bellani et al. 2016), ARDS is in fact still underrecognised, undertreated and, probably for these reasons, still associated with high mortality. We should also understand that ARDS is a heterogeneous syndrome, comprised of distinct phenotypes, different severity and different response to treatments. We need to recognise this and to adapt mechanical ventilation to individual patient conditions, particularly when there is a clear failure of the standard lung protective ventilation strategy. This means knowing the size of the lung that should be ventilated, to select the optimal tidal volume, and knowing if the lung is recruitable and how much can be recruited, to optimize the level of PEEP. Modern technology allows us to have this information at the bedside.
As Timothy G. Buchman wrote: “Precision medicine for critical illness and injury is desirable and achievable. Part of precision lies in standardization of practice. Part of the precision lies in individualization of care” (Buchman 2016). These all come together as the right care for the right patient, every time.
Take Home Points
- Individualized ventilation in ARDS can potentially improve outcome,
reduce treatment side effects and use resources better
- Recognition and diagnosis of ARDS needs to improve
- Differentiating and recognising the ARDS phenotypes is
important for treatment and outcome
- Protective ventilation is mandatory to reduce
ventilator-induced lung injury (VILI) and improve survival in ARDS patients
- Individualized ventilation should be based on: 1) recognizing
ARDS 2) assessing features of lung injury and its severity 3) individually
titrating tidal volume and PEEP, according to the lung volume 4) most
importantly, understanding the physiology behind mechanical ventilation
References:
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med, 342(18): 1301-8.
Briel M, Meade M, Mercat A et al. (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA, 303(9): 865-73.
Brower RG, Lanken PN, MacIntyre N et al. (2004) Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med, 351(4): 327-36.
Caironi P, Cressoni M, Chiumello D et al. (2010) Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med, 181(6): 578-86.
Calfee CS, Delucchi K, Parsons PE et al. (2014) Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med, 2(8): 611-20.
Dellamonica J, Lerolle N, Sargentini C et al. (2011) PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med, 37(10): 1595-604.
Ferguson ND, Fan E, Camporota L et al. (2012) The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med, 38(10): 1573-82.
Gattinoni L, Caironi P, Cressoni M et al. (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med, 354(17): 1775-86.
Gattinoni L, Marini JJ, Pesenti A et al. (2016) The "baby lung" became an adult. Intensive Care Med, 42(5): 663-73.
Maggiore SM, Jonson B, Richard JC et al. (2001) Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med, 164(5): 795-801.
Meade MO, Cook DJ, Guyatt GH et al. (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA, 299(6): 637-45.
Mercat A, Richard JC, Vielle B et al. (2008) Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA, 299(6): 646-55.
Richard JC, Brochard L, Vandelet P et al. (2003) Respective effects of end-expiratory and endinspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med, 31(1): 89-92.
Terragni PP, Rosboch G, Tealdi A et al. (2007) Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med, 175(2): 160-6.




















