A new non-invasive, image-based tracking of
stem cell signals in living animals could provide a strategy to arrest
pancreatic cancer growth, especially the most common pancreatic ductal
adenocarcinoma, which is extraordinarily lethal, with a 5-year survival rate of
just 6 percent. Chemotherapy treatments are poorly effective, in part due to a
high degree of drug-resistance to currently used regimens.
In a new study, published online on June 6 in Nature, researchers at University of California San Diego School of Medicine and Moores Cancer Centre, together with colleagues at Keio University, the University of Nebraska and Ionis Pharmaceuticals describe an innovative new model that not only allowed them to track drug resistance in vivo, but also revealed a new therapeutic target, which early testing suggests could provide a strategy to arrest pancreatic cancer growth.
In a collaboration that combined scientific and clinical expertise, principal investigators Tannishtha Reya, PhD, professor in the departments of Pharmacology and Medicine at UC San Diego School of Medicine, and Andrew Lowy MD, chief of surgical oncology in the Department of Surgery at UC San Diego Health and Moores Cancer Centre, worked with colleagues to develop a new “reporter” mouse model that enables non-invasive, image-based tracking of stem cell signals in living animals.
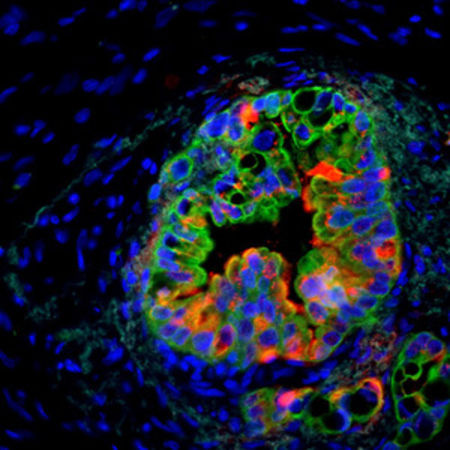
Using this strategy, the group showed that the stem cell gene Musashi (Msi) is a critical element in pancreatic cancer progression. In particular, the work revealed that Msi expression rises with cancer progression and that Msi expressing cells are key drivers of cancer growth, drug resistance and lethality.
Given the role of Msi in promoting aggressive disease, the investigators partnered with Robert MacLeod PhD, vice-president of oncology drug discovery at Ionis Pharmaceuticals, to develop next-generation antisense oligonucleotide (ASO) inhibitors against Msi. These inhibitors effectively targeted and blocked Msi expressing cells, resulting in halted tumour growth in animal models as well as in patient-derived cancer cells, which harbour more complex mutations and are uniformly drug-resistant.
Antisense inhibitors are synthetic nucleic acid drugs that can be designed to selectively bind to messenger RNA from the targeted, disease-linked gene, and inactivate it.
Reya said the findings could be broadly useful for studying cancer.
“Because Msi reporter activity can be visualised by live imaging,” said Reya, “these models can be used to track cancer stem cells within the tumour microenviroment, providing a real-time view of cancer growth and metastasis, and serving as a platform to test new drugs that may be better able to eradicate resistant cells.”
She also noted the targeting Msi in primary tumours significantly changed “the trajectory of progression by halting pancreatic cancer growth and improving survival,” inhibiting both cancer stem cells and other tumor cells. “This really highlights our ability to translate these findings and suggests that Msi antagonists could be a new strategy for targeting resistance to chemotherapy.”
Co-authors include: first authors Raymond G. Fox, and Nikki K. Lytle; Dawn V. Jaquish, Frederick D. Park, Takahiro Ito, Jeevisha Bajaj, Claire S. Koechlein, Bryan Zimdahl, Marcie Kritzik, Jason Sicklick, Donald Pizzo, Mark Valasek, Roman Sasik, and Miriam Scadeng all at UC San Diego; Masato Yano, Shinsuke Shibata, and Hideyuki Okana, Keio University School of Medicine, Japan; Janel Kopp, and Maike Sander, Sanford Consortium for Regenerative Medicine and UC San Diego; Paul M. Grandgenett, and Michael A. Hollingsworth, University of Nebraska Medical Center; and Youngsoo Kim, Ionis Pharmaceuticals, Carlsbad.