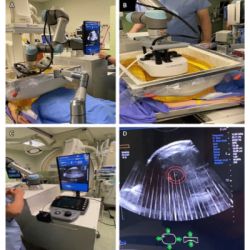
The new product is an important step forward in biopsy technology — allowing radiologists to better target lesions found during 3D MAMMOGRAPHY™ screening, as well as other screening modalities — with exceptional imaging, improved workflow and seamless, 360-degree access to the breast.
"As screening and diagnostic technology has advanced with the widespread use of 3D MAMMOGRAPHY™, it has become abundantly clear that prone biopsy technology was lagging behind," said Pete Valenti, Hologic's Division President, Breast and Skeletal Health Solutions. "We are excited to bring the Affirm™ prone biopsy system to the European market, and in doing so, mark one of the biggest steps forward in prone biopsy technology since the systems were first introduced over 20 years ago. A significantly improved imaging capabilityii and a streamlined workflow will provide healthcare providers with increased confidence for these critical procedures so that they can provide the best patient experience."
The Affirm™ prone biopsy system expands Hologic's breast biopsy portfolio, complementing the Company's Selenia® Dimensions® mammography system and Affirm™ upright biopsy system. This portfolio provides radiology facilities all the options necessary to provide minimally invasive breast biopsies for their patients without compromise.
Thousands of clinicians worldwide have trusted prone patient positioning for breast biopsy as it supports the patient stably throughout the procedure while isolating them from the biopsy needle. This provides a better overall patient experience.
The new system will be unveiled at the annual European Congress of Radiology meeting in Vienna, Austria from March 2-6. ECR is the largest medical imaging meeting in Europe and attracts participants from across the world.
Hologic is offering several tomosynthesis-related events at ECR:
- ECR Satellite Symposium, "The Use of 3D MAMMOGRAPHY™
Exams in High Volume Screening," on Friday, March 4 from 2:00 p.m. – 3:00 p.m. [I assume this is CET; need to include.] - Seven Educational Sessions from March 3-5 focusing on the clinical value of vacuum-assisted 3D™ breast biopsy.
- Seven Educational Sessions from March 3-5 on the clinical use of 3D MAMMOGRAPHY™ exams with a focus on reading and interpreting cases.
In addition to the Affirm™ prone biopsy system, other innovative products to be featured at ECR include:
- The Horizon™ bone densitometry (DXA) system, a single platform for osteoporosis and obesity assessment;
- I-View™ software, the first and only Contrast-Enhanced 2D Mammography exam that can be combined with tomosynthesis;
- Low dose 3D MAMMOGRAPHY™ exams with C-View™ software, delivering superior clinical performance to 2D mammography for all breast typesiii,iv,v in a faster, more comfortable and lower dose 3D MAMMOGRAPHY™ exam;
- Affirm™ upright biopsy system for Selenia® Dimensions®, the proven 3D™ breast biopsy system providing superior upright biopsy performancevi,vii and
- Eviva® and ATEC® breast biopsy systems, designed to conduct minimally invasive biopsy procedures under stereotactic, tomosynthesis, ultrasound or MRI guidance.
For additional information on the Affirm™ prone biopsy system, please visit www.affirmpronebiopsy.com, and for registration details, email [email protected].
The Affirm™ prone biopsy system is not currently for sale in the United States.
Source & Image Credit : Hologic
References:
[i] Compared to the MultiCare® Platinum system.
[ii] Compared to existing dedicated prone biopsy systems
[iii] FDA PMA submission P080003/S001 physician labeling
[iv] Skaane P, Bandos A, Eben E, et al. "Two-View Digital Breast Tomosynthesis Screening with Synthetically Reconstructed Projection Images: Comparison with Digital Breast Tomosynthesis with Full-Field Digital Mammographic Images" Radiology. 2014 Jun;271:3, 655-663. Epub 2014 Jan 24.
[v] Zuley M, Guo B, Catullo V, et al. "Comparison of Two-dimensional Synthesized Mammograms versus Original Digital Mammograms Alone and in Combination with Tomosynthesis Images." Radiology. 2014 Jun;271(3):664-71. Epub 2014 Jan 21.
[vi] Schrading S, Martine D, Dirrichs T, et al. "Digital Breast Tomosynthesis-guided Vacuum-assisted Breast Biopsy: Initial Experiences and Comparison with Prone Stereotactic Vacuum-assisted Biopsy." Radiology. 2014 Nov 11.
[vii] Smith A, Sumpkin J, Zuley M, et al. "Comparison of Prone Stereotactic vs. Upright Tomosynthesis Guided Vacuum Assisted Core Breast Biopsies." (paper presented at the annual meeting for the Radiological Society of North America. Chicago, Il, November 2014).