ICU Management & Practice, ICU Volume 15 - Issue 3 - 2015
What Is New?
Following a severe burn injury, an overwhelming systemic inflammatory response with capillary leak syndrome is initiated, resulting in a combined hypovolaemic and septic shock (Malbrain et al. 2014a). Numerous articles regarding burn resuscitation have been published over the last decades; however, there is no universal consensus on how to achieve adequate resuscitation whilst avoiding the adverse effects of excessive resuscitation. As a consequence, a dynamic fluid protocol including also active de-resuscitation is needed (Cordemans et al. 2012a; Cordemans et al. 2012b; Kushimoto et al. 2012; Kirkpatrick et al. 2013).
Historical Background
Although burn wounds and burn-related deaths have been part of human history, fluid resuscitation management is relatively new, dating back less than a century. In 1921, landmark research was performed by Frank Underhill following the New Haven Rialto Theater fire ( Underhill 1930). Following the Coconut Grove fire in 1942, Cope and Moore stated in a series of articles on thermal injury that wound oedema contributed to the burn shock, and the proposed volume for resuscitation was based on the patient’s body weight and the severity of the burn, the so-called “body weight burn budget” (Cope and Moore 1947). In 1952 Evans postulated a formula for fluid volumes based on total burned surface area (TBSA) and also introduced colloids in burn resuscitation (Evans et al. 1952). This formula would become the standard until the 1960s (Haynes et al. 1955). At that time Baxter and Shires developed their pivotal formula at the Parkland Memorial Hospital, which has lasted decades as the gold standard for fluid resuscitation in acute burn care across the world (Baxter and Shires 1968). The formula advocates 4ml crystalloids per kg per % of TBSA per 24h, of which half is given during the first eight hours (Baxter and Shires 1968). Resuscitation fluids are guided by diuresis (target 1 ml/kg/hour) and increased with steps of 25%. During the second 24 hours of resuscitation, colloids are allowed, and resuscitation volume is adapted according to diuresis (with a gradual decrease if diuresis is adequate).
Fluid Creep
Over the last 15 years, however, multiple centres have reported excess fluid administration (Cartotto and Zhou 2010; Faraklas et al. 2012; Saffle 2007; Shah et al. 2003; Strang et al. 2014). This fluid excess often leads to “resuscitation morbidity”, a group of complications linked to fluid overload, such as pulmonary oedema (due to capillary leak and increased extravascular lung water), delayed wound healing, delayed recovery of gastrointestinal function (with ileus), limb compartment syndrome, orbital compartment syndrome, intraabdominal hypertension (IAH) and abdominal compartment syndrome (ACS) leading to multiple organ failure (Malbrain et al. 2014a; Kirkpatrick et al. 2013; Malbrain et al. 2014b; Pruitt 2000; Sullivan et al. 2006; Ball et al. 2006).
This discrepancy between the predicted and the administered fluid is known as “fluid creep”, a term brought to life by Basil Pruitt (2000). There are different hypotheses regarding the phenomenon, although its cause remains uncertain. One interesting hypothesis is that of “opioid creep”, a term introduced by Sullivan et al. (2004). Over the last fifteen years, a lot of attention has been paid to the phenomenon of fluid creep, and the awareness of morbidity caused by resuscitation has grown significantly. Efforts should therefore be made to avoid “futile loading” with excessive amounts of crystalloids and the role of colloids in early resuscitation needs to be further investigated (Lawrence et al. 2010).
Recommendations for fluid
resuscitation, resuscitation endpoints and suggestions for treatment algorithms
are listed in Table 1.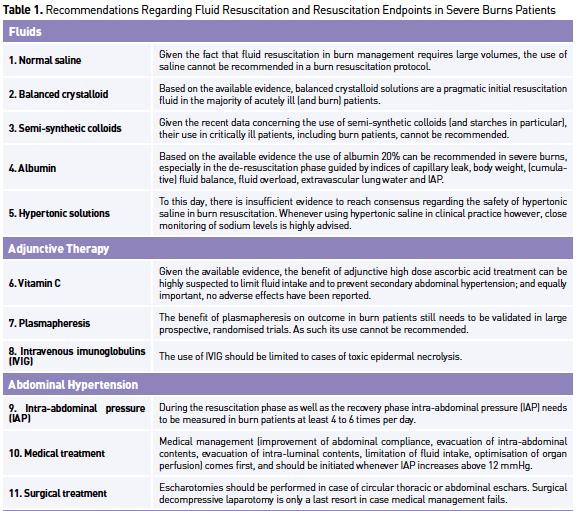
Types of Resuscitation Fluid
Crystalloids
Crystalloids are aqueous solutions of mineral salts, which are freely permeable across membranes. In 1882 a “normal” saline solution (NaCl 0.9%, 154 mEq/L) was developed by Hamburger, believing it was the sodium concentration of the plasma (Awad et al. 2008). The most well-known adverse effect is hyperchloremic metabolic acidosis after infusion of large volumes saline and, as such, normal saline cannot be recommended as resuscitation fluid in severe burns (Stephens and Mythen 2000). The “balanced” or “physiologic” solutions such as lactated Ringer’s, Hartmann’s solution or Plasmalyte add the anion bicarbonate in the form of lactate, acetate or gluconate; it provides a strong ion difference which is feasible from an acid-base perspective ( Van Regenmortel et al. 2014; McDermid et al. 2014; Young et al. 2014). From the beginning of burn resuscitation until present, most resuscitation formulas advocate the use of balanced crystalloid solutions and as such they are a pragmatic initial choice in severe burns patients. One observational study reported lower Sequential Organ Failure (SOFA) scores in severely burned patients resuscitated with Ringer’s acetate (Gille et al. 2014).
Colloids
Specifically in burn resuscitation, the use of colloids in the first 24 hours has been controversial since it was thought that the existing capillary leak would allow large molecules to leak into the extravascular space and exert an osmotic pull increasing the formation of oedema (Baxter 1974). During the last decades colloids have been omitted from many resuscitation formulas. In the last fifteen years however, renewed interest in colloids has arisen, instigated by the awareness of morbidity related to resuscitation and fluid creep. Until recently, the low molecular weight hydroxyethyl starch (HES) solutions were widely used as a resuscitation fluid in critically ill ICU, surgical and burn patients. However, recent trials such as Crystalloid versus Hydroxyethyl Starch Trial (CHEST), Scandinavian Starch for Severe Sepsis/Septic Shock (6S) and Effects of Voluven on Hemodynamics and Tolerability of Enteral Nutrition in Patients With Severe Sepsis (CRYSTMAS) showing increased mortality and higher rate of renal replacement therapy have raised alarming conclusions regarding the safety of HES solutions (Myburgh et al. 2012; Perner et al. 2012; Guidet et al. 2012). This led to the recommendation of the Pharmacovigilance Risk Assessment Committee (PRAC) against the use of HESsolutions in patients with sepsis, burn injuries or critically ill patients, because of increased kidney injury and mortality (Coriat et al. 2014).
Albumin
Albumin is a natural plasma protein that contributes most to intravascular oncotic pressure in humans. The most common solution is 4 or 5% albumin in saline. It is a relatively expensive solution and its availability may be limited in some countries. Although albumin resuscitation has been used with some reservation, especially in the acute phase of burn resuscitation, trials provide promising data regarding the use of albumin as an adjunctive in burn resuscitation (Lawrence et al. 2010; Cochran et al. 2007).
Hypertonic saline
Hypertonic saline has been used for decades in burn resuscitation; theoretically, it expands the circulating volume by an intravascular water shift (Duchesne et al. 2015). This will decrease tissue oedema and lower the rate of complications claimed by proponents. In the 1970s, studies concluded that hypertonic saline indeed reduces the volume needed for burn resuscitation (Monafo et al. 1973; Moylan et al. 1973).
Adjunctive Therapy
Vitamin C
In the 1990s, Matsuda et al. were able to reduce fluid requirements and oedema formation during burn resuscitation in dogs and guinea pigs using high-dose ascorbic acid treatment (Matsuda et al. 1991; Matsuda et al. 1992). A few years later they reproduced in a prospective, randomised study the proposed beneficial effects of high dose ascorbic acid in humans (Tanaka et al. 2000).
Plasmapheresis
In the 1980s a retrospective study described plasmapheresis treatment in patients who failed to respond to conventional therapy. This therapeutic response was characterised by a sharp decrease in fluid requirements from a mean of 260% above the predicted hourly volume to within calculated requirements by 2.3 hours following plasma exchange (Warden et al. 1983).
Role of Abdominal Hypertension
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are major complications in burn patients leading to multi-organ dysfunction and death, requiring specific strategies to prevent, monitor, diagnose and treat these complications (Malbrain et al. 2015). In 1994 it was reported that the incidence of ACS was linked with the extent of burn injury. This relationship between TBSA and development of ACS was confirmed in other studies (Strang et al. 2014; Oda et al. 2006; Ivy et al. 2000; Kirkpatrick et al. 2009). Typically, ACS occurs when TBSA is greater than 60%; however, patients with a lower TBSA may also develop IAH/ACS (Oda et al. 2006). Whether the development of IAH and ACS is iatrogenic or can be avoided is not clear. In 2000 Ivy stated that a volume administration of > 250ml/kg in the first 24 hours is a risk factor for ACS; this fluid quantity is known as the Ivy index (Ivy et al. 2000).
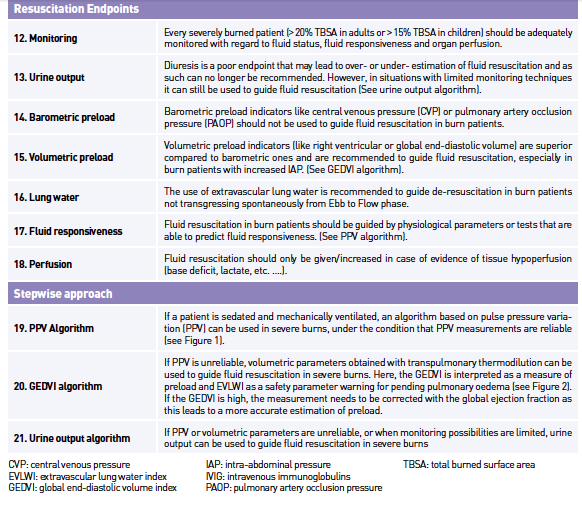
Resuscitation Targets and Endpoints
When resuscitating burn patients, clinicians need to evaluate the optimal amount of fluid to be given. The clinical interpretation of haemodynamic status can be very difficult in burn patients, which is problematic because there is a risk for inadequate organ perfusion as well as a risk of over-resuscitation.
Urine Output
Urine output has classically been the primary endpoint to guide resuscitation in burn care; popular opinion was that a target diuresis of 0.5ml/kg/h in adults and 1ml/kg/h in a paediatric population should be pursued. This endpoint, however, has been doubted in studies. In a retrospective review (Dries and Waxman 1991), there was no correlation between urine output and invasively derived physiologic variables; moreover urine output was unable to identify fluid responders after a fluid challenge. Other studies also suggest the inconsistency of urine output as a resuscitation target (Shah et al. 2003; Pruitt 2000), perhaps even contributing to the phenomenon of fluid creep.
Barometric Preload
Studies in recent years have questioned the efficacy of pulmonary artery occlusion pressure (PAOP) and central venous pressure (CVP), as endpoints for resuscitation, as these parameters do not correlate with ventricular filling pressures and ventricular end-diastolic volumes (Marik 2011; Michard and Teboul 2002). In a systematic review, Marik found a very poor relationship between CVP and blood volume, as well as the inability to predict the haemodynamic response to a fluid challenge, making CVP and PAOP obsolete as standardised endpoints for fluid resuscitation. Its use should be reserved for specific indications (Marik et al. 2008).
Volumetric Preload
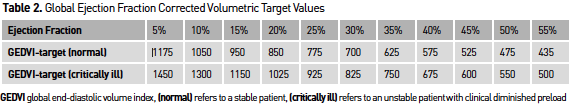
Advances in technology such as transpulmonary thermodilution allow the monitoring of preload in static volumetric indices such as global enddiastolic volume (GEDV) and extravascular lung water (EVLW). Numerous studies have shown that these volumetric indices represent preload more precisely in comparison to urine output (Csontos et al. 2008; Sanchez et al. 2013) or cardiac filling pressures (Lichtwarck-Aschoff et al. 1996; Malbrain et al. 2010), where during the early resuscitation phase, hypovolaemia might not be reflected by blood pressure or urine output (Sanchez et al. 2013). Correcting these volumetric preload parameters by measures of ejection fraction, this further improves the ability of these parameters to assess changes in preload over time as presented in Table 2 (Malbrain et al. 2010). The EVLW together with the pulmonary vascular permeability index (PVPI) can be used to determine the presence of lung oedema, which can be very useful as a safety parameter during resuscitation (Sanchez et al. 2013), in patients with inhalation injury or in guiding fluid de-resuscitation if a patient fails to proceed to the “flow” phase (Sanchez et al. 2014a; Cordemans et al. 2012a; Cordemans et al. 2012b).
Fluid Responsiveness
To determine the benefit of fluid administration, several clinical and haemodynamic tests can be used. As mentioned above, CVP does not predict fluid responsiveness (Michard and Teboul 2002; Marik et al. 2008), but studies have shown that dynamic parameters, obtained by invasive arterial monitoring and pulse contour analysis, such as pulse pressure variation (PPV) and stroke volume variation (SVV) are highly predictive of fluid responsiveness (Marik et al. 2009) in mechanically ventilated patients.
Holistic Approach: Introduction of a New Protocol
In an attempt to effectively guide fluid resuscitation in burn patients in the future, whilst avoiding deleterious effects of over-resuscitation, a multimodal protocol using a modified formula and multiple endpoints is suggested.
Fluid resuscitation is initiated in adults with >20% TBSA and children with >15% TBSA. A modified Parkland formula is suggested; a balanced crystalloid is given at 2 ml/kg/%TBSA in the first 24 hours in combination with albumin 20% at 0.2 ml/ kg/%TBSA; half of the total dose of crystalloids and colloids is given in the first eight hours, the other half between 8-24 hours. During the next 24 hours a calcium-free balanced salt solution is given at 0.75 ml/kg /%TBSA in combination with albumin 20% at 0.075 ml/kg /%TBSA. These resuscitation rates are fixed, and fluids are gradually decreased after the first 24 hours of burn shock resuscitation. Throughout the resuscitation, basic fluid needs are supplemented in the form of a glucose containing solution at 30ml/kg/24h; enteral nutrition, if given, is subtracted from this basic fluid administration total. Fluids are changed or adapted throughout resuscitation according to biochemical analysis (base excess (BE), lactate, etc.) and concomitant medical conditions (see algorithms as discussed further). During the first 24 hours, resuscitation fluids as calculated above are kept at constant rate and when needed fluid boluses can be given. De-resuscitation (with gradual decrease in resuscitation fluids) is only started after the first 24 hours. Extra albumin 20% can be administered based on serum levels of albumin (target 30 g/L) or colloid oncotic pressure (COP, target 16-18 mmHg).
PPV Algorithm
Different endpoints in combination with lactate and BE can be used in order to guide fluid resuscitation. If a patient is sedated and mechanically ventilated, PPV is used, whenever reliable. The subsequent PPV algorithm is presented in Figure 1; however, the clinician needs to check whether the patient has conditions leading to incorrect interpretation of PPV (Hofkens et al. 2015).
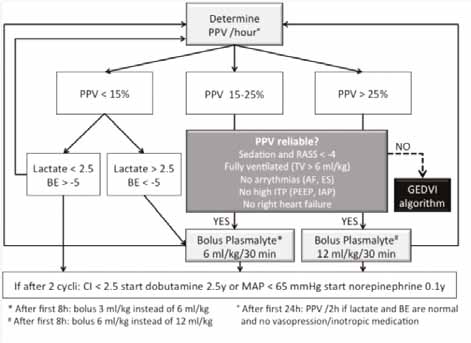
mechanically ventilated and PPV is reliable fluid resuscitation is guided by the PPV algorithm.
GEDVI Algorithm
If PPV is unreliable, volumetric parameters are measured with the use of transpulmonary thermodilution. Here the GEDVI is interpreted as a measure of preload and EVLWI as a safety parameter warning for pending pulmonary oedema. The subsequent GEDVI algorithm is presented in Figure 2. Correct interpretation of volumetric parameters needs to be done in relation to the presence or not of other conditions (like valvulopathy, catheter position, extracorporeal circuit, etc.) (Hofkens et al. 2015).
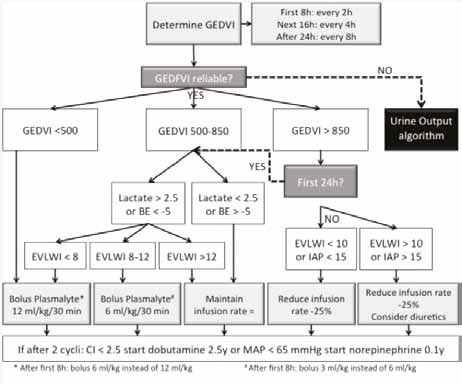
Figure 2. Global end-diastolic volume index algorithm to guide resuscitation in severely burned patients.If PPV is unreliable and the patient has a PiCCO catheter and GEDVI is not reliable fluid resuscitation is guided by the GEDVI Algorithm.
Urine Output Algorithm
If PPV or volumetric parameters are unreliable, or when monitoring possibilities are limited; urine output (UO) can be used to guide fluid resuscitation. The subsequent UO algorithm is presented in Figure 3. The importance of measuring IAP needs to be underlined when using urine output as a resuscitation target, as IAH and ACS decrease urine output.
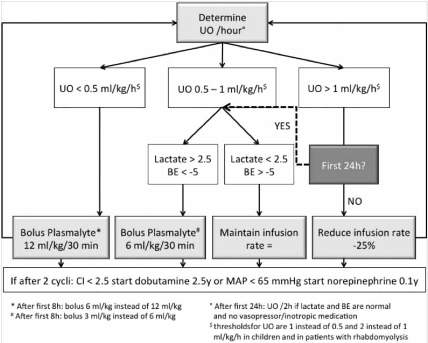
Figure 3. Urine output algorithm to guide resuscitation in severely burned patients. If the patient has no PiCCO catheter (or GEDVI is not reliable) and PPV is not reliable, fluid resuscitation is guided by the UO algorithm.
Conclusions
Burn resuscitation keeps evolving and new trends develop. Over the last fifteen years, much attention was given to avoid over-resuscitation and subsequent morbidity and mortality; fluid creep is recognised by nearly all physicians involved in burn care.
Efforts should be made to avoid excess crystalloid administration by re-thinking resuscitation protocols. Evidence suggests that the addition of a colloid such as albumin 20% may decrease fluid requirements, and may potentially reduce resuscitationrelated morbidity; however, the use of colloids in burn resuscitation continues to be a great source of controversy and discussion.
Endpoints of burn resuscitation should be redefined. The traditional UO target does not represent preload accurately; however, barometric preload parameters also seem to lack this ability. Evidence suggests that advanced haemodynamic monitoring with pulse contour analysis (PPV) and transpulmonary thermodilution (GEDVI) may provide superior endpoints to prevent underresuscitation, while EVLWI can be used as a safety parameter to avoid over-resuscitation and to guide the de-resuscitation process.
Acknowledgements
MLNGM is member of the medical advisory board of Pulsion Medical Systems (Maquet Getinge Group). The other authors have no possible conflicts of interest related to the content of this paper. This article is a selection of a full review paper published under the Open Access CC BY Licence in Anaesthesiology and Intensive Therapy.
References:
Ball CG, Kirkpatrick AW, Karmali S et al. (2006) Tertiary abdominal compartment syndrome in the burn injured patient. J Trauma, 61(5): 1271-3.
Baxter CR, Shires T (1968) Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci, 150(3): 874-94.
Baxter CR (1974) Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg, 1(4): 693-703.
Cartotto R, Zhou A (2010) Fluid creep: the pendulum hasn't swung back yet! J Burn Care Res, 31(4): 551-8.
Cochran A, Morris SE, Edelman LS et al. (2007) Burn patient characteristics and outcomes following resuscitation with albumin. Burns, 33(1): 25-30.
Cope O, Moore FD (1947) The Redistribution of Body Water and the Fluid Therapy of the Burned Patient. Ann Surg, 126(6): 1010-45.
Cordemans C, De Laet I, Van Regenmortel N et al. (2012a) Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak and fluid balance. Ann Intensive Care, 2(Suppl 1): S1.
Cordemans C, De Laet I, Van Regenmortel N et al. (2012b) Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive Care, 2 (Suppl 1): S15.
Coriat P, Guidet B, de Hert S et al. (2014) Counter statement to open letter to the Executive Director of the European Medicines Agency concerning the licensing of hydroxyethyl starch solutions for fluid resuscitation. Br J Anaesth, 113(1): 194-5.
Csontos C, Foldi V, Fischer T et al. (2008) Arterial thermodilution in burn patients suggests a more rapid fluid administration during early resuscitation. Acta Anaesthesiol Scand, 52(6): 742-9.
Dries DJ, Waxman K (1991) Adequate resuscitation of burn patients may not be measured by urine output and vital signs. Crit Care Med,19(3): 327-9.
Duchesne JC, Kaplan LJ, Balogh ZJ et al. (2015) Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensive Ther, 47(2): 143-55.
Evans EI, Purnell OJ, Robinett PW et al. (1952) Fluid and electrolyte requirements in severe burns. Ann Surg, 135(6): 804-17.
Faraklas I, Cochran A, Saffle J (2012) Review of a fluid resuscitation protocol: "fluid creep" is not due to nursing error. J Burn Care Res, 33(1): 74-83.
Gille J, Klezcewski B, Malcharek M et al. (2014) Safety of resuscitation with Ringer's acetate solution in severe burn (VolTRAB)--an observational trial. Burns, 40(5): 871-80.
Guidet B, Martinet O, Boulain T et al. (2012) Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care,16(3): R94.
Haynes BW Jr., Martin MM, Purnell OJ (1955) Fluid, colloid and electrolyte requirements in severe burns. I. An analysis of colloid therapy in 158 cases using the Evans formula. Ann Surg, 142(4): 674-9; discussion, 679-81.
Hofkens PJ, Verrijcken A, Merveille K et al. (2015) Common pitfalls and tips and tricks to get the most out of your transpulmonary thermodilution device: results of a survey and state-of-the-art review. Anaesthesiol Intensive Ther, 47(2): 89-116.
Ivy ME, Atweh NA, Palmer J et al. (2000) Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma, 49(3): 387-91.
Kirkpatrick AW, Ball CG, Nickerson D et al. (2009) Intraabdominal hypertension and the abdominal compartment syndrome in burn patients. World J Surg, 33(6): 1142-9.
Kirkpatrick AW, Roberts DJ, De Waele J et al. (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med, 39(7): 1190-206.
Kushimoto S, Taira Y, Kitazawa Y et al. (2012) The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care, 16(6): R232.
Lawrence A, Faraklas I, Watkins H et al. (2010) Colloid administration normalizes resuscitation ratio and ameliorates "fluid creep". J Burn Care Res, 31(1): 40-7.
Lichtwarck-Aschoff M, Beale R, Pfeiffer UJ (1996) Central venous pressure, pulmonary artery occlusion pressure, intrathoracic blood volume, and right ventricular end-diastolic volume as indicators of cardiac preload. J Crit Care, 11(4): 180-8.
Malbrain ML, De Keulenaer BL, Oda J et al. (2015) Intra-abdominal hypertension and abdominal compartment syndrome in burns, obesity, pregnancy, and general medicine. Anaesthesiol Intensive Ther, 47(3): 228-40.
Malbrain ML, De Potter TJ, Dits H et al. (2010) Global and right ventricular end-diastolic volumes correlate better with preload after correction for ejection fraction. Acta Anaesthesiol Scand, 54(5): 622-31.
Malbrain ML, Marik PE, Witters I et al. (2014a) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther, 46(5): 361-80.
Malbrain ML, Roberts DJ, Sugrue M et al. (2014b) The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther, 46(5):433-50.
Marik PE, Baram M, Vahid B (2008) Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest, 134(1): 172-8.
Marik PE, Cavallazzi R, Vasu T et al. (2009) Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Critical Care Med, 37(9): 2642-7.
Marik PE (2011) Surviving sepsis: going beyond the guidelines. Ann Intensive Care, 1(1):17.
Matsuda T, Tanaka H, Hanumadass M et al. (1992) Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil, 13(5): 560-6.
Matsuda T, Tanaka H, Williams S et al. (1991) Reduced fluid volume requirement for resuscitation of third-degree burns with high-dose vitamin C. J Burn Care Rehabil, 12(6): 525-32.
McDermid RC, Raghunathan K, Romanovsky A et al. (2014) Controversies in fluid therapy: Type, dose and toxicity. World J Crit Care Med, 3(1): 24-33.
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest, 121(6): 2000-8.
Monafo WW, Chuntrasakul C, Ayvazian VH (1973) Hypertonic sodium solutions in the treatment of burn shock. Am J Surg, 126(6): 778-83.
Moylan JA Jr., Reckler JM, Mason AD Jr. (1973) Resuscitation with hypertonic lactate saline in thermal injury. Am J Surg, 125(5): 580-4.
Myburgh JA, Finfer S, Bellomo R et al. (2012) Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med, 367(20): 1901-11.
Oda J, Yamashita K, Inoue T et al. (2006) Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burns, 32(2): 151-4.
Perner A, Haase N, Guttormsen AB et al. (2012) Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med, 367(2): 124-34.
Pruitt BA Jr. (2000) Protection from excessive resuscitation: "pushing the pendulum back". J Trauma, 49(3): 567-8.
Saffle JI (2007) The phenomenon of "fluid creep" in acute burn resuscitation. J Burn Care Res, 28(3): 382-95.
Sánchez M, Garcí a-de-Lorenzo A, Herrero E et al. (2013) A protocol for resuscitation of severe burn patients guided by transpulmonary thermodilution and lactate levels: a 3-year prospective cohort study. Crit Care, 17(4): R176.
Shah A, Kramer GC, Grady JJ et al. (2003) Meta-Analysis of Fluid Requirements for Burn Injury 1980-2002. J Burn Care Rehabil,152(24): S118.
Stephens RC, Mythen MG (2000) Saline-based fluids can cause a significant acidosis that may be clinically relevant. Crit Care Med, 28(9): 3375-7.
Strang SG, Van Lieshout EM, Breederveld RS (2014) A systematic review on intra-abdominal pressure in severely burned patients. Burns, 40(1): 9-16.
Sullivan SR, Ahmadi AJ, Singh CN et al. (2006) Elevated orbital pressure: another untoward effect of massive resuscitation after burn injury. J Trauma, 60(1): 72-6.
Sullivan SR, Friedrich JB, Engrav LH et al. (2004) "Opioid creep" is real and may be the cause of "fluid creep". Burns, 30(6): 583-90.
Tanaka H, Matsuda T, Miyagantani Y et al. (2000) Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg, 135(3): 326-31.
Underhill FP (1930) The significance of anhydremia in extensive superficial burns. JAMA, 95(12): 852-7.
Van Regenmortel N, Jorens PG, Malbrain ML (2014) Fluid management before, during and after elective surgery. Curr Opin Crit Care, 20(4): 390-5.
Warden GD, Stratta RJ, Saffle JR et al. (1983) Plasma exchange therapy in patients failing to resuscitate from burn shock. J Trauma, 23(10): 945-51.
Young JB, Utter GH, Schermer CR et al. (2014) Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg, 259(2): 255-62.





















