ICU Management & Practice, ICU Volume 14 - Issue 2 - Summer 2014
Authors

Xavier Monnet, MD, PhD
Professor in Intensive Care Medicine
Medical Intensive Care Unit Bicêtre Hospital
Paris-South University Hospitals
Assistance Publique-Hôpitaux de, Paris
Le Kremlin-Bicêtre, France
[email protected]

Jean-Louis Teboul, MD
Professor
Faculty of Medicine, Paris-Sud University
Le Kremlin-Bicêtre, France
[email protected]
Introduction
In case of acute circulatory failure, fluid is administered with the expectation that it will increase cardiac output. Nevertheless, this can occur only if cardiac output is dependent upon cardiac preload, ie if both ventricles operate on the ascending limb of the cardiac function curve (see Figure 1) (Monnet et al. 2013). If no test is used to predict fluid responsiveness, volume expansion results in the expected increase in cardiac output in only half of patients (Michard et al. 2002). Thus, fluid responsiveness should be detected before deciding to administer volume expansion or not. This should avoid fluid overload, which is an independent predictor of mortality in patients with septic shock (Vincent et al. 2006) and/or ARDS (Jozwiak et al. 2013).
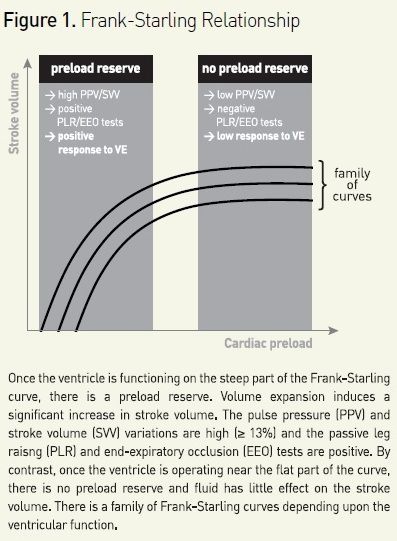
For predicting fluid responsiveness, ‘static’ markers of cardiac preload have been used for many years: central venous pressure, pulmonary artery occlusion pressure, left ventricular end-diastolic dimensions at echocardiography, for instance. However, a very large number of studies clearly demonstrate that such markers of preload are unable to predict fluid responsiveness (Monnet et al. 2013). This is mainly due to the physiological fact that a given value of preload could correspond to either a large or a negligible response of cardiac output to fluid administration, depending upon the slope of the Frank-Starling curve (see Figure 1). Today, using such static markers of preload for deciding to administer fluid or not should be definitely abandoned (Marik et al. 2013). Alternatively, a ‘dynamic approach’ allows assessment of preload dependency by observing the effects on cardiac output of changes in cardiac preload induced by various tests.
Respiratory Variations of Stroke Volume: a High Level of Evidence
During mechanical ventilation each insufflation decreases venous return and, if the right ventricle is preload-dependent, reduces the right ventricular outflow. Increase in right ventricular afterload induced by increased lung volume contributes to this reduction of right ventricular outflow. After the transit of blood through the lungs the left ventricular preload decreases. In the case of conventional ventilation, this should occur at expiration. If the left ventricle is also preload-dependent, the left ventricular stroke volume transiently decreases in response. Hence, a cyclic variation of stroke volume under mechanical ventilation indicates preload-dependency of both ventricles (Michard et al. 2000).
Surrogates of Stroke Volume
The arterial pulse pressure was the first index to be used to predict fluid responsiveness through its respiratory variation (Michard et al. 2000). Many other surrogates of stroke volume have been developed since, such as stroke volume estimated by pulse contour analysis, aortic blood flow measured by oesophageal Doppler, aortic blood flow with echocardiography and amplitude of the plethysmography signal with pulse oximetry (Monnet et al. 2013).
Limitations of the Respiratory Variations of Stroke Volume to Predict Fluid Responsiveness
It is important to always remember that the respiratory variation of haemodynamic signals cannot be used in some specific conditions. First, in case of spontaneous breathing activity, even in an intubated patient, variations of stroke volume relate more to the respiratory irregularity than to preload dependence (Heenen et al. 2006, Monnet et al. 2006). The second obvious limitation is the presence of cardiac arrhythmias. The third limitation refers to ARDS. In such a case, the low tidal volume (De Backer et al. 2005) and/or the low lung compliance (Monnet et al. 2012), which reduces the transmission of changes in alveolar pressure to the intrathoracic structures, diminish the amplitude of the ventilation-induced changes of intravascular pressure. This should result in false negatives for the prediction of fluid responsiveness by pulse pressure variation (PPV). Open chest surgery, a low ratio of heart rate over respiratory rate (corresponding in fact to respiratory rates at 40 breaths/minute or more) or intraabdominal hypertension are other circumstances in which PPV will be unreliable to predict fluid responsiveness (Marik et al. 2011).
The End-Expiratory Occlusion (EEO) Test
During mechanical ventilation each insufflation increases the intrathoracic pressure and impedes venous return. Thus, interrupting the respiratory cycle at end-expiration inhibits the cyclic impediment in venous return and transiently increases cardiac preload (see Figure 1). It has been demonstrated that if a 15 second EEO increases the arterial pulse pressure or the pulse contour-derived cardiac index by more than 5%, the response of cardiac output to a 500 mL saline infusion can be predicted with good sensitivity and specificity (Monnet et al. 2009).
Beyond its simplicity, the main advantage of the EEO test is that it exerts its haemodynamic effects over several cardiac cycles and thus remains valuable in case of cardiac arrhythmias (Monnet et al. 2009). It can also be used in patients with spontaneous breathing activity, unless a too marked triggering activity interrupts the 15-second EEO.
The ‘Mini’ Fluid Challenge
The disadvantage of the ‘classical’ fluid challenge is that it consists of the infusion of 300-500 mL of fluid (Vincent et al. 2006). This may obviously contribute to fluid overload if the test is negative.
A new method of ‘mini fluid challenge’ has been proposed as an alternative (Muller et al. 2011). It consists of administering 100 mL of colloid over 1 minute and observing the effects of this ‘mini’ increase in cardiac preload on stroke volume, measured by the subaortic velocity time index using transthoracic echocardiography (Muller et al. 2011).
A strong limitation of the mini fluid challenge may be that, even in cases of preload-dependency, such a small volume infusion will unavoidably induce only small changes in cardiac output. Thus, this test requires a very precise technique for measuring cardiac output.
The Passive Leg Raising (PLR) Test
In a subject lying down, raising the legs from the horizontal position passively transfers a significant volume of blood from the lower part of the body toward the cardiac chambers. This induces a reversible increase in cardiac preload (Monnet et al. 2006). Several studies have reported that the increase in cardiac output induced by this ‘endogenous’ volume challenge allows prediction of fluid responsiveness with reliability (Cavallaro et al. 2010). Interestingly, since the test exerts its effects over several cardiac cycles and since it is not related to heart-lung interactions, PLR remains a good predictor of fluid responsiveness in patients with spontaneous breathing activity, cardiac arrhythmias or ARDS (Monnet et al. 2006, Monnet et al. 2009).
Importantly, PLR must be started from the strict semi-recumbent position and not from the horizontal supine position. This mobilises blood coming not only from the inferior limbs but also from the large splanchnic compartment, and substantially increases sensitivity of the PLR test (Jabot et al. 2009).
Another important point concerns the method that can be used for measuring the changes in cardiac output during PLR (Monnet et al. 2008). Firstly, these effects cannot be assessed by observing the simple arterial pressure. Indeed, the PLRinduced changes in arterial pulse pressure are less accurate than the PLR-induced changes in cardiac output or stroke volume. Secondly, the PLR test requires a real-time measurement of cardiac output allowing tracking of haemodynamic changes in the timeframe of PLR effects, ie 30-90 seconds. This can be done by aortic blood flow measured by oesophageal Doppler, pulse contour analysis-derived cardiac output, cardiac output measured by bioreactance, subaortic velocity measured by echocardiography and even end-tidal carbon dioxide (Monnet et al. 2013).
Conclusions
Several tests have been developed to detect volume responsiveness before administering fluid. The choice between them should be made depending on the clinical setting and the patient’s condition. Using such tests should avoid administering fluid if it is not haemodynamically effective. This should avoid fluid overload, a condition that increases mortality of critically ill patients, particularly in cases of sepsis and/or lung impairment.
Acknowledgements
XM and J-L T are members of the Medical Advisory Board of Pulsion Medical Systems
References:
Cavallaro F, Sandroni C, Marano C et al. (2010) Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med, 36: 1475-83.
De Backer D, Heenen S, Piagnerelli M et al. (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med, 31: 517-23.
Heenen S, De Backer D, Vincent JL (2006) How can the response to volume expansion in patients with spontaneous respiratory movements be predicted? Crit Care, 10: R102.
Jabot J, Teboul JL, Richard C, Monnet X (2009) Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med, 35: 85-90.
Jozwiak M, Silva S, Persichini R et al. (2013) Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med, 41: 472-80.
Marik PE, Cavallazzi R (2013) Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med, 41: 1774-81.
Marik PE, Monnet X Teboul JL (2011) Hemodynamic parameters to guide fluid therapy. Ann Intensive Care, 1: 1.
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest, 121: 2000-8.
Michard F, Boussat S, Chemla D et al. (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med, 162: 134-8.
Monnet X, Teboul JL (2013) Assessment of volume responsiveness during mechanical ventilation: recent advances. Crit Care, 17: 217.
Monnet X, Teboul JL (2008) Passive leg raising. Intensive Care Med, 34: 659-63.
Monnet X, Bataille A, Magalhaes E et al. (2013) End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med, 39: 93-100.
Monnet X, Bleibtreu A, Ferré A et al. (2012) Passive leg raising and endexpiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med, 40: 152-7.
Monnet X, Osman D, Ridel C et al. (2009) Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med, 37: 951-6.
Monnet X, Rienzo M, Osman D et al. (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med, 34: 1402-7.
Muller L, Toumi M, Bousquet PJ et al. (2011) An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology, 115: 541-7.
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med, 34: 1333-7.
Vincent JL, Sakr Y, Sprung CL et al. (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med, 34: 344-53.




















