Prudent strategies to optimise nutrition for the critically ill child, improving long-term outcomes, and preserving quality of life.
Introduction
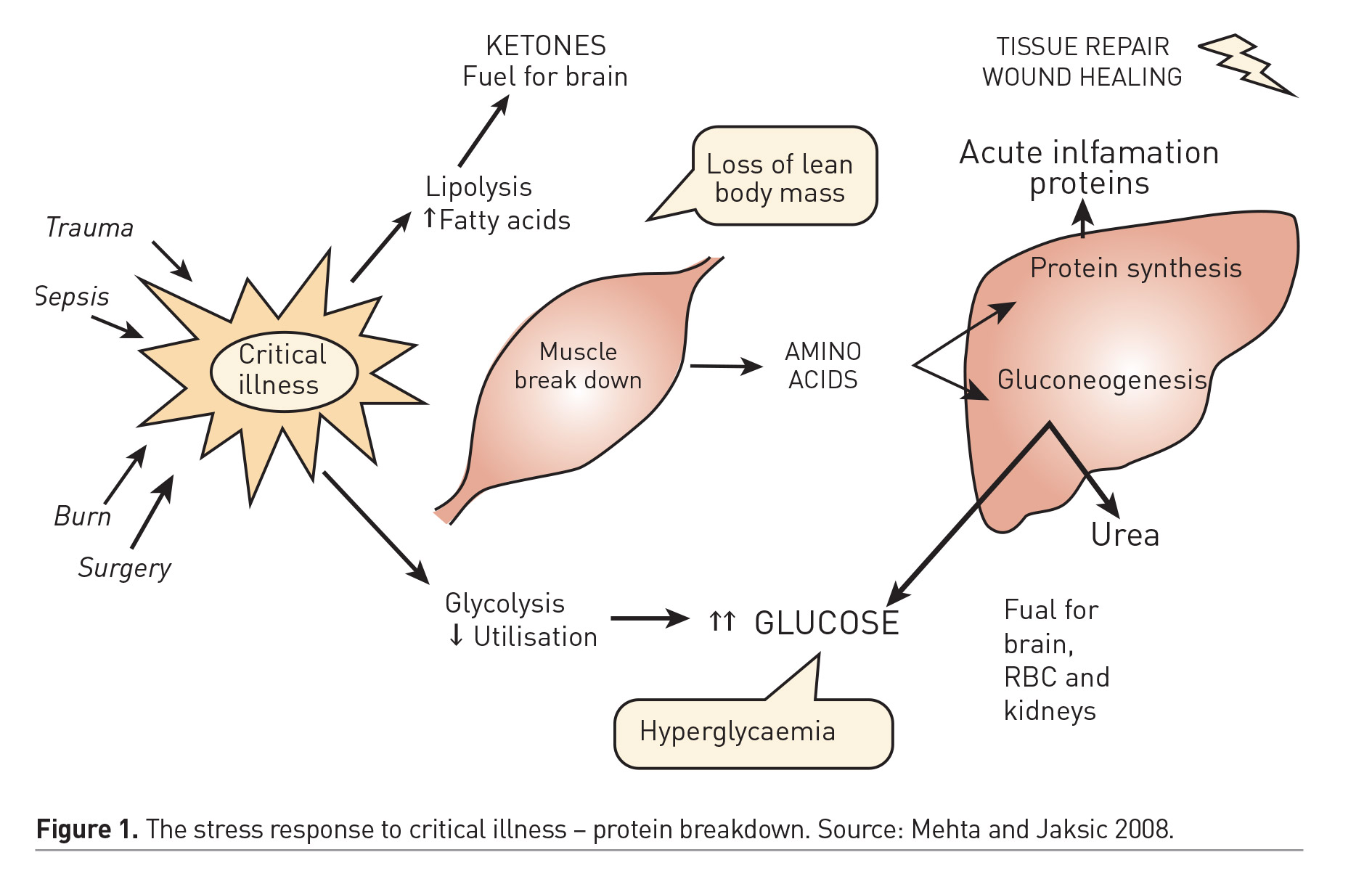
Optimal delivery of nutrients to the critically ill patient might prevent nutritional deterioration and expedite recovery. Prospective cohort studies have demonstrated the independent association between nutritional status and important clinical outcomes (de Souza Menezes et al. 2012). Furthermore, failure to provide adequate nutrient intake during critical illness has been associated with deterioration of nutritional status and poor clinical outcomes (Mehta et al. 2012; Mehta et al. 2015). Hence, optimal nutrition therapy is an important component of the care of critically ill children and an area of ongoing interest and inquiry. The impact of specific nutrition strategies on clinical outcomes have not been adequately demonstrated in randomised clinical trials. As a result, there is some uncertainty about the optimal timing, route and dose of nutrition therapy during critical illness, and practice patterns at the bedside vary widely across PICUs worldwide. This era of increased interest but scant evidence is fertile for myths and dogma arising from observational studies, poorly designed trials with limited external validity, and expert opinion. A basic understanding of the metabolic demands from critical illness might help develop a sound nutrition strategy. Figure 1 depicts the key aspects of the metabolic stress response to critical illness in humans (Mehta and Jaksic 2008). The energy burden and protein loss that are imposed by this response are relevant targets that may be addressed by optimal delivery of these macronutrients to support the individual and prevent lean body mass loss during critical illness. Investigations over past decades have highlighted that energy requirements may be lower than expected, and the energy expenditure estimations by standard equations are inaccurate, often leading to overfeeding. Protein breakdown is the principal feature of the stress response to critical illness and may result in lean body mass loss that is undesirable. Optimal energy and protein delivery, while preventing overfeeding, may help offset protein losses and preserve muscle mass and long-term function in critically ill patients.
Nutrition Therapy – Key Questions
There are 3 fundamental questions related to nutrition during acute critical illness:
- What is the optimal dose for macronutrients (energy, protein) and the role for supplemental micronutrients?
- What is the best route for nutrient delivery: a) enteral nutrition - EN; b) parenteral nutrition - PN; or c) EN with supplemental PN.
- What is the best timing for EN initiation and when (early vs. late) should PN be initiated as a supplement if EN is not feasible or insufficient?
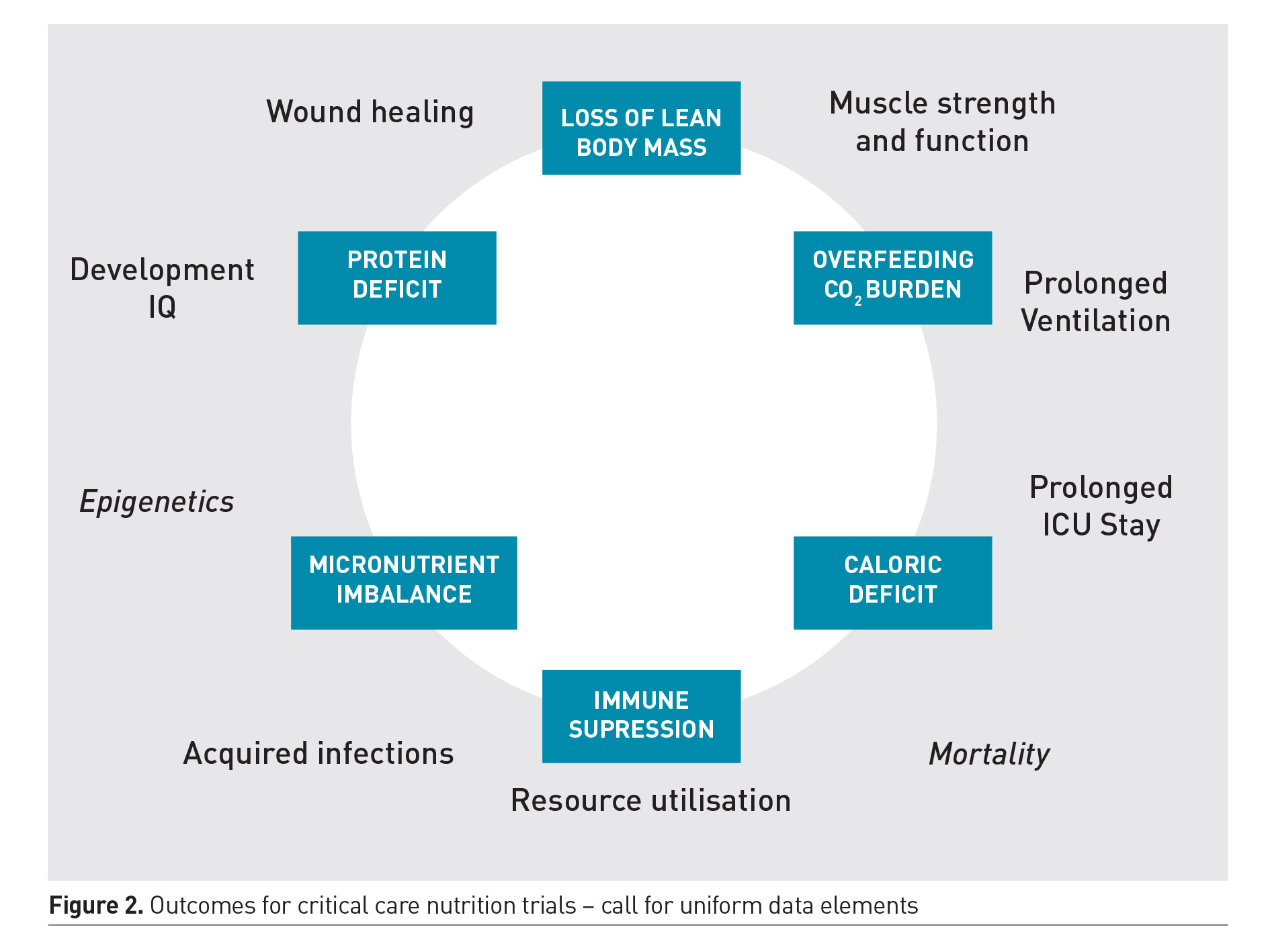
Optimal nutrition therapy involves careful prescription of the dose of energy, protein and micronutrients; delivered at appropriate time during the illness course; via the most appropriate and safe route. These decisions are often interlinked and the optimal strategy may vary between individuals, dependent on the nature and severity of illness and its metabolic effects, nutritional status and gastrointestinal dysfunction. Unfortunately, the few trials that do exist on this subject have explored a one size fits all strategy applied uniformly to a vastly heterogeneous patient population. Some of the trials have limited external validity and practical questions related to bedside practice during critical illness remain unanswered. The optimal design that allows careful examination of these interrelated concepts remains elusive. While some of these questions will require rigorous examination by randomised allocation of distinct therapies; the quest to determine one uniform strategy that would apply to all PICU patients is quixotic and must be abandoned. Future trials must employ innovative and more meaningful study designs to account for the interplay between dose, route and timing of nutrient delivery. These trials must include relevant clinical outcomes, and a core set of well-defined data elements must be employed to allow results from different trials to be compared. There are ongoing efforts to develop such a core set that include meaningful outcomes beyond survival. Figure 2 shows the common surrogate and functional outcomes of interest for future nutritional trials. Preservation of muscle mass and function is the most important short-term outcome that may be associated with improved functional and clinical outcomes from critical illness. The role of nutrition along with other non-nutritional strategies in preserving muscle mass and function is therefore an area of ongoing investigation.
Evaluating Emerging Evidence for Nutrition Therapy - Guidelines
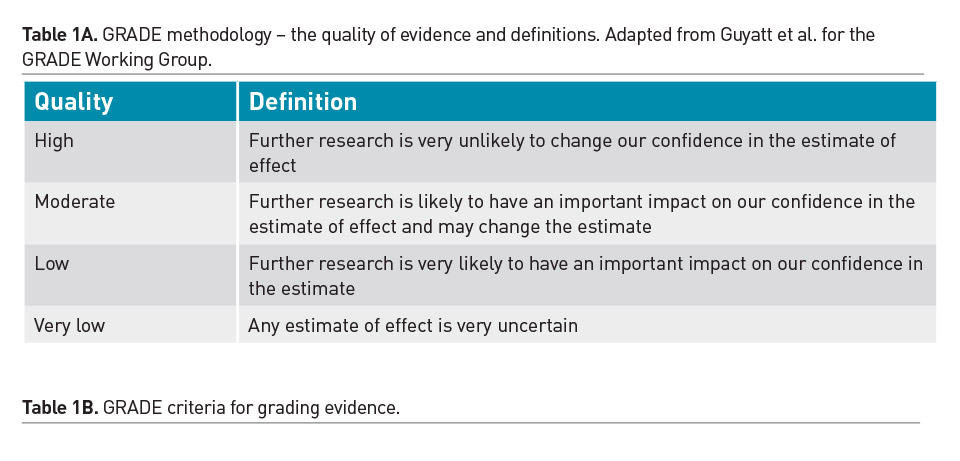
Individual practitioners must carefully assess the merits and validity of each emerging study and determine the applicability of its results to their patients. Randomised controlled trials (RCT) are recognised as the strongest clinical evidence, however weaknesses in the design or implementation of an RCT will decrease the quality of that evidence. Furthermore, single trials are often refuted in clinical medicine and premature adoption of practices based on limited evidence should be avoided. Due to the scarcity of robust RCTs, a majority of nutritional practices in the PICU have been adopted based on observational data from cohort studies or expert opinion. Regular review of the literature and translation of the cumulative evidence into practical recommendations is essential. There have been significant advances in the process of systematic assessment and cumulative incorporation of emerging trial results into guidelines. The GRADE methodology for review of literature is used to develop best practice recommendations and is described in Table 1 (Druyan et al. 2012; Guyatt et al. 2008).
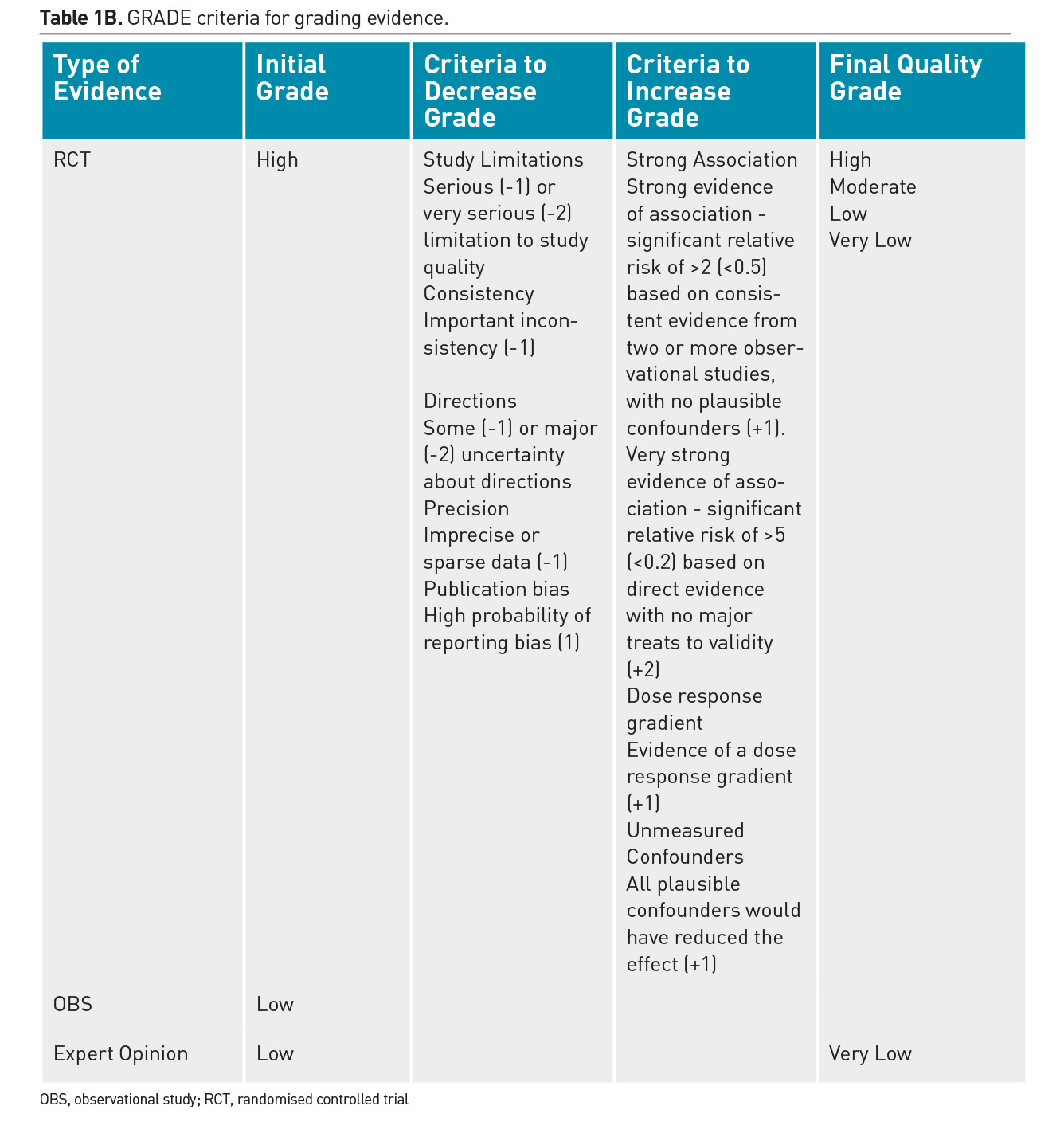
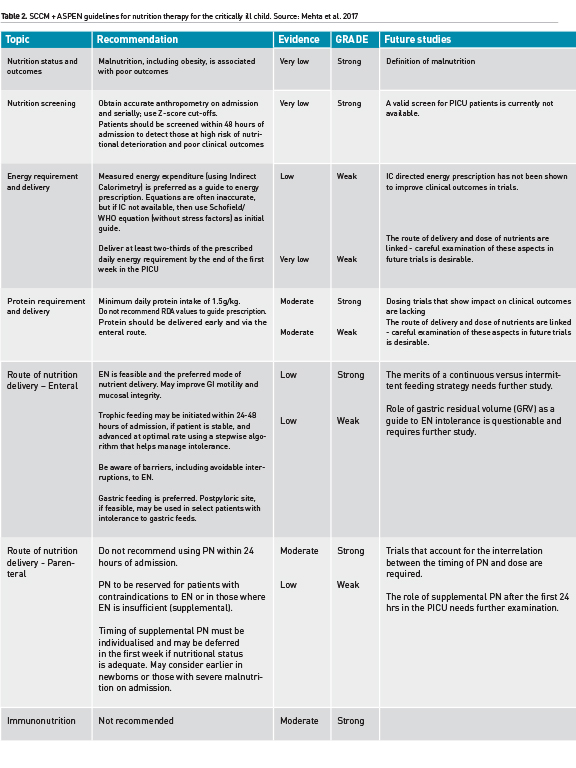
The American Society for Parenteral & Enteral Nutrition (ASPEN) and the Society of Critical Care Medicine (SCCM) have recently published an exhaustive review of evidence and generation of evidence tables that were then translated by a multidisciplinary group of experts into practice recommendations for nutrition therapy in the paediatric intensive care unit (Mehta et al. 2017). These guidelines must be revised and updated every few years to reflect emerging evidence. Table 2 summarises key recommendations from these guidelines.
A Pragmatic Approach to Nutrition in the PICU
Step 1: Nutrition Prescription
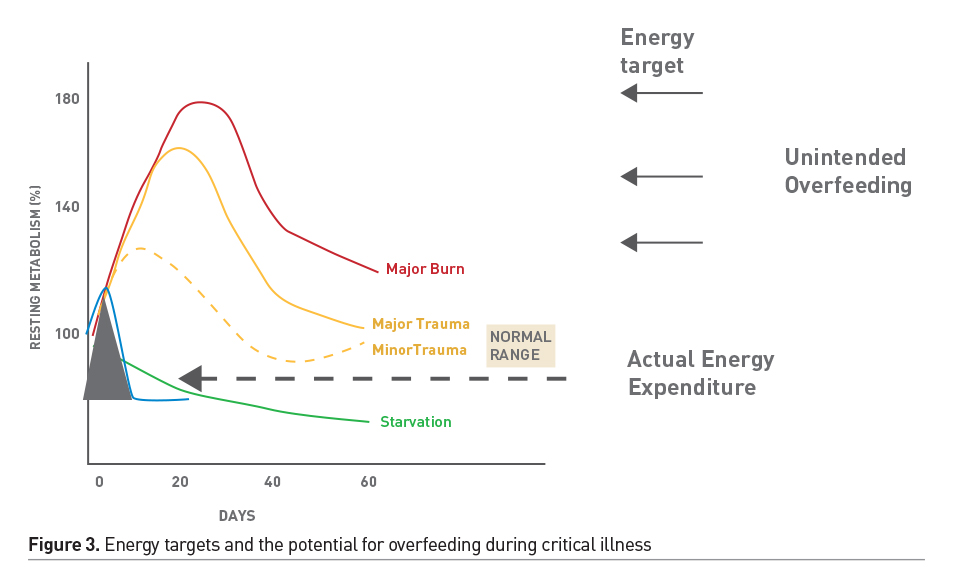
Nutrition screening helps identify patients who are at a high risk of nutritional deterioration and poor outcome; it allows early allocation of limited nutritional resources where they might have the most impact. However, a valid screen for critically ill children is not available. Detailed nutritional assessment allows detection of existing nutritional deficiencies and specific disease related nutritional needs. Energy requirement may be highly variable and based on the nature of illness/injury. Indirect calorimetry (IC), during steady-state conditions, is the gold standard method for accurate energy expenditure assessment (Mehta et al. 2017). However, IC may not be feasible in a large subset of children due to technological and physiologic hurdles. When IC is not feasible or available, estimates of energy expenditure using standard equations plus stress factors to adjust for illness severity and activity have been used to guide energy prescription. However, equation estimates are inaccurate and may result in unintended underfeeding or overfeeding of energy, which may impact patient outcomes (White et al. 2000; Ladd et al. 2018). These equations were developed in populations of healthy children and therefore may not reflect energy expenditure in critically ill children. Sedated and mechanically ventilated children, in thermoneutral environments in modern ICUs, may have significant reduction in energy expenditure. These patients may be at a risk of overfeeding when prescriptions are guided by estimates of energy requirements, especially if stress factors are incorporated (Figure 3). In the absence of IC, Schofield/WHO equations may be used as a guide (Mehta et al. 2017). Stress or correction factors should only be applied after careful consideration of metabolic status in individual cases. In a large cohort study, delivery of 2/3 of the prescribed amount was associated with improved outcomes. Hence, guidelines recommend 2/3 (rather than full) prescription as appropriate target for energy delivery in the first week of critical illness.
Large observational study data have shown strong association between increased protein delivery (percentage of the prescribed target) and lower 28-day mortality. Previous trials have also shown that protein supplementation increases the likelihood of achieving a positive protein balance (Mehta et al. 2015). However, the optimal dose of protein that is associated with improved clinical outcomes has not been studied using randomised controlled trials. Furthermore, the secondary analysis from a recent RCT examining timing of PN, implicated amino acids as the macronutrient responsible for the adverse effects of an aggressive approach to early initiation of PN (Fivez et al. 2016). Therefore, a well-designed dosing study of protein in the first week of critical illness is desirable.
Overall, the nutrition prescription in critically ill children must be individualised for each patient. The energy dose should preferably be guided by measurements of energy expenditure. Optimal protein dosing and timing are recently being questioned, although most observational and trials data suggest that a minimal protein intake of 1.5g/kg/day is associated with improved outcomes (Mehta et al. 2017).
Step 2: Optimal Nutrition Delivery - Enteral Route
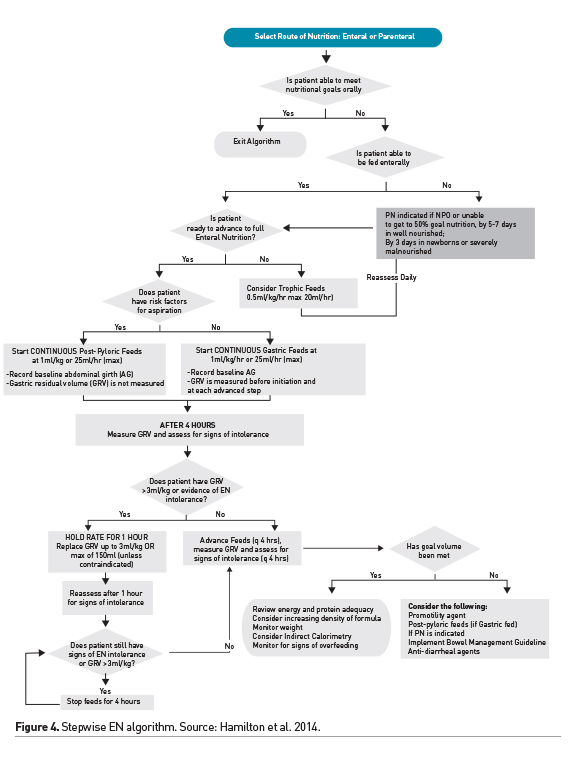
Enteral nutrition is preferred and is feasible in a majority of critically ill children. Small volume, nonnutritive, feeding in the gut has benefits and may be initiated within 24-48 hours of admission in children with a functioning gastrointestinal tract, initiated soon after haemodynamic stabilisation. Stepwise protocols have been shown to optimise advancement of EN, guiding rates of feeding and assisting in the diagnosis and management of EN intolerance. Figure 4 shows an example of a stepwise EN advancement protocol (Hamilton et al. 2014). Interruptions for procedures, intolerance to EN and fluid restriction are common barriers to achieving goal nutrient delivery via the enteral route. Attention to these barriers in the ICU and efforts to decrease, when possible, fasting times in critically ill children are desirable (Mehta et al. 2010). EN intolerance remains challenging as we update our definition and management strategies. Elevated gastric residual volume (GRV) is routinely used in a majority of ICUs as a surrogate for intolerance. However, this practice of stopping feeds based on a threshold GRV value has been challenged and it may not be used as a singular marker of EN intolerance (Tume et al. 2017). Improving our understanding of the mechanisms of gastrointestinal dysmotility during critical illness and developing strategies to ameliorate it are desirable. Overall, we have made significant strides in achieving safe and optimal delivery of enteral nutrition in the critically ill child, and strategies for optimising EN remain an area of great interest and ongoing investigation in critical care.
Step 3: Optimal Nutrition Delivery - Parenteral Route
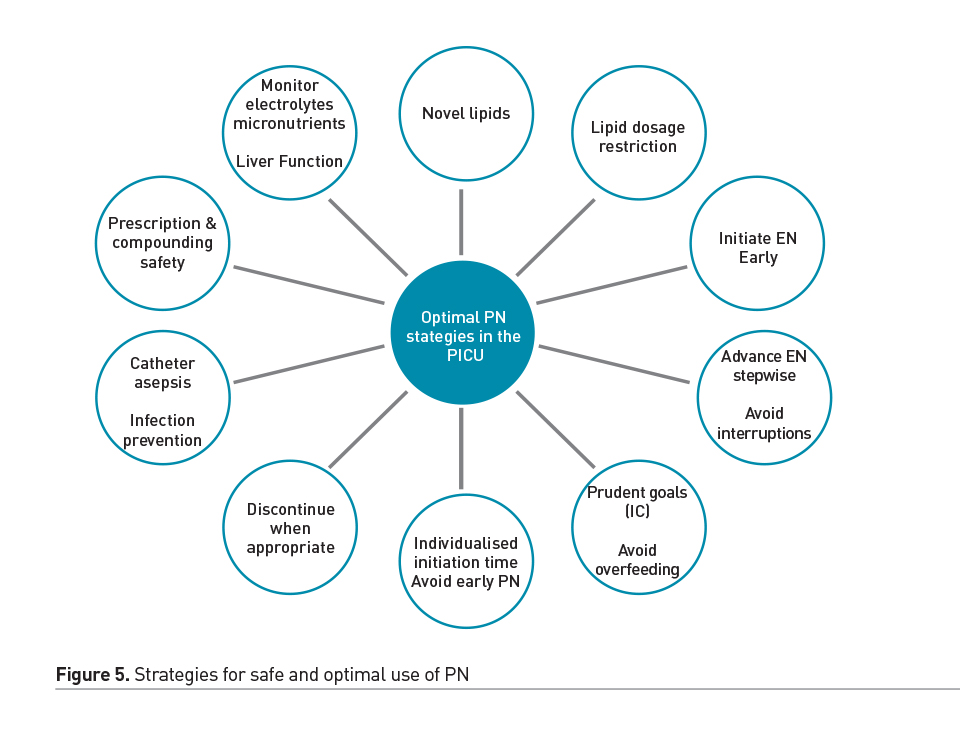
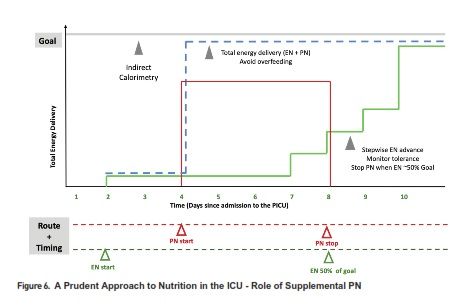
However, in many patients, EN is either contraindicated, not tolerated, and therefore insufficient to meet the nutritional needs alone. Parenteral nutrition (PN) emerged in the 20th century as a life-saving therapy in such circumstances (Wilmore et al. 1968). Over the years, attention to PN safety and prudent PN strategies have allowed us to utilise the benefits of this mode of nutrition delivery, while balancing against its potential complications. Central catheter associated blood stream infection and PN associated cholestasis and liver disease are important considerations in children dependent on PN. Therefore, the timing of PN as a supplemental nutrition delivery mode has been an area of controversy and investigation in adult and paediatric critical care. In a recent randomised controlled trial, an aggressive early PN approach (initiated within 24 hours of admission to the PICU) was shown to be associated with longer PICU stay and increased likelihood of acquired infections, compared to a late PN strategy (initiated after 7 days) (Fivez et al. 2016). Despite the debate surrounding the study design and its external validity, it clearly demonstrated that PN use soon after admission to the PICU is not beneficial as a uniform strategy, and in most cases PN may be deferred during the first week in the PICU, while providing adequate micronutrients and advancing EN as tolerated. In particular, the ill effects of the early PN strategy may also be related to the potential overfeeding in this group, compared to those that were randomised to the late PN strategy (Mehta et al. 2016). PN may be initiated sometime during the first week, to avoid hypoglycaemia and cumulative nutrient deficiencies, especially in newborns or those with severe malnutrition at baseline. A prudent approach to advancing PN as supplement or alternative to insufficient EN in select cases, by Day 4 in the ICU, was shown to improve infection rates in adults when compared to a late PN initiation strategy (Heidegger et al. 2013). Figure 5 summarises some of the strategies that have been employed to reduce complications and side effects in cases of chronic PN dependence. Overall, careful assessment to detect high risk patients, emphasis of early initiation and advancement of EN using algorithms, and prudent use of PN for select cases with particular attention to avoiding overfeeding, is a reasonable strategy for utilising the optimal route of nutrient delivery during acute critical illness. Figure 6 illustrates elements of a prudent EN and PN strategy during the first week of ICU admission.
Summary and Future Directions
Malnutrition, including obesity, negatively impacts outcomes from critical illness. Critically ill children do not always respond to critical illness with hypermetabolism and often have decreased energy requirements. Overfeeding, from inaccurate estimates of energy requirement, must be avoided. Indirect calorimetry is a critical tool that guides energy prescription in the ICU. The ‘less is more concept’ is most applicable to energy delivery during early acute critical illness, when endogenous energy production, anabolic resistance and risk of overfeeding preclude the benefits of an early and aggressive nutrition strategy. On the other hand, protein breakdown is a principal feature of critical illness metabolism, and optimal protein delivery to offset losses may help preserve lean body mass during prolonged critical illness. Both energy and protein targets must be individually determined for each patient; a ‘one size fits all’ approach for dose, timing and route of nutrient delivery is not reasonable. EN remains the preferred route of nutrient delivery in critically ill children. Early initiation, stepwise advancement with careful assessment for safety and management of intolerance, and avoidance of unnecessary interruptions are features of a prudent EN strategy. Aggressive use of early PN is harmful and must be avoided. A pragmatic approach for individualised timing of PN as a supplement to insufficient EN, aiming for at least 2/3rd of the prescribed energy goal by the end of the first week of illness is recommended. Optimal PN strategies may offset its side effects and allow effective use in select patients.
Future trials will need to demonstrate the impact of nutrition strategies on long-term functional outcomes in patients. These trials will need innovative designs with high external validity and testing of the nuances of nutrition delivery. Adoption of common/uniform data elements will allow comparisons between the impact of nutritional strategies on meaningful outcomes. Muscle mass and function preservation is one of the key goals of nutrition during critical illness, and a variety of techniques to measure muscle mass and function are being investigated. There is significant interest in exploring other therapies such as early mobilisation, physical rehabilitation, exercise, and muscle stimulation to help achieve this goal (Choong et al. 2018). The role of nutrition in combination with these non-nutritive therapies must be explored (Wischmeyer et al. 2017). The future of nutrition lies in pragmatic individualised therapies that help children recover from critical illness with minimal impact on their long-term development, function and quality of life.
Key points
- Critically ill children do not always respond to critical illness with hypermetabolism and often have decreased energy requirements.
- Overfeeding, from inaccurate estimates of energy requirement, must be avoided - indirect calorimetry is a critical tool that must be used to guide energy prescription in the ICU.
- Enteral nutrition is preferred and is feasible in a majority of critically ill children.
- Parenteral nutrition use soon after admission to the PICU is not beneficial as a uniform strategy, and may be deferred during the first week in the PICU.
- Muscle mass and function preservation are key goals of nutrition during critical illness using optimal nutritional therapies in combination with non-nutritive strategies.
Abbreviations
ASPEN American Society for Parenteral & Enteral Nutrition
EN Enteral nutrition
GRV Gastric Residual Volume
IC Indirect calorimetry
ICU Intensive Care Unit
OBS Observational Study
PICU Paediatric Intensive Care Unit
PN Parenteral nutrition
RCT Randomised controlled trials
SCCM Society of Critical Care Medicine
Choong K, Canci F, Clark H et al. (2018) Practice Recommendations for Early Mobilization in Critically Ill Children.J Pediatr Intensive Care, 7(1):14-26.
de Souza Menezes F, Leite HP, Koch Nogueira PC (2012) Malnutrition as an independent predictor of clinical outcome in critically ill children. Nutrition, 28(3):267-270.
Druyan ME, Compher C, Boullata JI et al. (2012) Clinical Guidelines For the Use of Parenteral and Enteral Nutrition in Adult and Pediatric Patients: applying the GRADE system to development of A.S.P.E.N. clinical guidelines. JPEN J Parenter Enteral Nutr, 36(1):77-80.
Fivez T, Kerklaan D, Mesotten D et al. (2016) Early versus Late Parenteral Nutrition in Critically Ill Children. N Engl J Med, 24;374(12):1111-22.
Guyatt GH, Oxman AD, Vist G, et al for the GRADE Working Group (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ, 336:924-926.5
Hamilton S, McAleer DM, Ariagno K et al. (2014) A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals. Pediatr Crit Care Med, 15(7):583-589.
Heidegger CP, Berger MM, Graf S et al. (2013) Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet, 381(9864):385-93.
Ladd AK, Skillman HE, Haemer MA et al. (2018) Preventing Underfeeding and Overfeeding: A Clinician's Guide to the Acquisition and Implementation of Indirect Calorimetry. Nutr Clin Pract, 33(2):198-205.
Mehta NM, Skillman HE, Irving SY et al. (2017) Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. Pediatr Crit Care Med, 18(7):675-715.
Mehta NM (2016) Parenteral Nutrition in Critically Ill Children. N Engl J Med, 24;374(12):1190-2.
Mehta NM, Bechard LJ, Zurakowski D et al. (2015) Adequate enteral protein intake is inversely associated with 60-d mortality in critically ill children: a multicenter, prospective, cohort study. Am J Clin Nutr, 102(1):199-206.
Mehta NM, Bechard LJ, Cahill N et al. (2012) Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study. Crit Care Med, 40(7):2204-2211.
Mehta NM, McAleer D, Hamilton S et al. (2010) Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr, 34(1):38-45.
Mehta N, Jaksic T (2008) The critically ill child. In: Duggan C, Watkins JB,Walker WA, editors. Nutrition in pediatrics. 4th edition. Hamilton, Ontario, Canada: BC Decker: 663–73.
Tume LN, Bickerdike A, Latten L et al. (2017) Routine gastric residual volume measurement and energy target achievement in the PICU: a comparison study. Eur J Pediatr, 176(12):1637-1644.
White MS, Shepherd RW, McEniery JA (2000) Energy expenditure in 100 ventilated, critically ill children: improving the accuracy of predictive equations. Crit Care Med, (7):2307-12.
Wilmore DW, Dudrick SJ (1968) Growth and development of an infant receiving all nutrients exclusively by vein. JAMA, 203(10):860-4.
Wischmeyer PE, Puthucheary Z, San Millán I et al. (2017) Muscle mass and physical recovery in ICU: innovations for targeting of nutrition and exercise. Curr Opin Crit Care, 23(4):269-278.