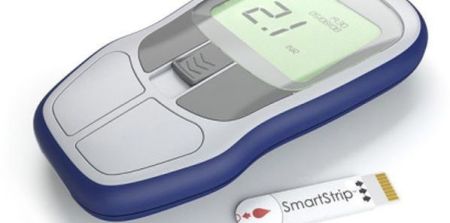
Microvisk Ltd.'s (North Wales, UK) first-of-its kind medical device – a disposable diagnostic strip based on a Micro-Electro-Mechanical System (MEMS) – has been accredited for European sales, Bionow reported.
“The achievement of CE marking of a medical diagnostic device based on a tiny MEMS sensor is a major milestone for Microvisk – and opens up further possibilities for this pioneering technology," said Jim Thurlow, CEO of Microvisk. The CE mark indicates mandatory compliance with all applicable EU Directives for diagnostic medical devices, effectively clearing products for sale in the European Economic Area, including the UK.
The company said its microcantilever sensor technology determines blood clotting speed from a finger prick sample, with results displayed on its compact CoagMax® handheld reader. Initially, the CoagMax® PT/INR system is to be used for monitoring patients taking anti-coagulants such as Warfarin, to minimise risks from life-threatening blood clots and to help manage correct dosage, according to the report.
Rapid Testing in Portable Format
The system, incorporating the MEMS technology on a disposable test strip, enables rapid testing in an easy-to-use, portable format. With the CoagMax® PT/INR system, patients can quickly and conveniently test blood clotting just as diabetics test for glucose, Microvisk explained.
About 10 to 12 million people in the Western world take anti-coagulants to combat the risk of blood clotting conditions, including stroke, heart attack and DVT. Patients on anti-coagulants (or blood thinners) need regular tests to confirm correct dosage levels, and successful trials by technology companies are fuelling an increasing trend for self-testing at home, led by Germany and the USA.
Microvisk noted that its platform technology is "unique in medical diagnostics as a robust, solid state system with the simplicity to revolutionise point-of-care and home testing." The PT/INR strip measures fluid viscosity, combining accurate, dependable lab quality results with exceptional ease of use.
“Bringing MEMS technology into the medical device market is a real step change, unlocking the potential of advanced technology to create smaller, simpler-to-use devices for the growing healthcare diagnostics market,” Thurlow said. Based on Microvisk's estimates, the potential EU market for the device could reach US $350 million by 2023.
CoagMax® and its disposable strip are clearly distinct from competitors’ existing technology which, according to Microvisk, relies on older, more complex optical analysis or chemical reaction-based measurements.
Eliminates Need for Lab Testing
Taking the CoagMax® meter to the finger reduces patient time and inconvenience for clinic attendance, while eliminating the need for a laboratory. Requiring only 8 ul of blood – less than competing systems – also means less pain for patients, Microvisk said.
The disposable strip uses the standard international PT (Prothrombin Time) or INR (International Normalised Ratio) test, by using a drop of a patient’s whole blood from a finger prick. The sensors detect blood changes to a gel-like substance and evaluate whether viscosity is within acceptable range for the patient’s medication.
Beyond medical diagnostics, Microvisk’s technology platform has future potential for numerous fluid viscosity testing applications – with oils and foodstuffs being prime examples, according to industry analysts.
Source: Bionow.co.uk
Image Credit: Medgadget.com
“The achievement of CE marking of a medical diagnostic device based on a tiny MEMS sensor is a major milestone for Microvisk – and opens up further possibilities for this pioneering technology," said Jim Thurlow, CEO of Microvisk. The CE mark indicates mandatory compliance with all applicable EU Directives for diagnostic medical devices, effectively clearing products for sale in the European Economic Area, including the UK.
The company said its microcantilever sensor technology determines blood clotting speed from a finger prick sample, with results displayed on its compact CoagMax® handheld reader. Initially, the CoagMax® PT/INR system is to be used for monitoring patients taking anti-coagulants such as Warfarin, to minimise risks from life-threatening blood clots and to help manage correct dosage, according to the report.
Rapid Testing in Portable Format
The system, incorporating the MEMS technology on a disposable test strip, enables rapid testing in an easy-to-use, portable format. With the CoagMax® PT/INR system, patients can quickly and conveniently test blood clotting just as diabetics test for glucose, Microvisk explained.
About 10 to 12 million people in the Western world take anti-coagulants to combat the risk of blood clotting conditions, including stroke, heart attack and DVT. Patients on anti-coagulants (or blood thinners) need regular tests to confirm correct dosage levels, and successful trials by technology companies are fuelling an increasing trend for self-testing at home, led by Germany and the USA.
Microvisk noted that its platform technology is "unique in medical diagnostics as a robust, solid state system with the simplicity to revolutionise point-of-care and home testing." The PT/INR strip measures fluid viscosity, combining accurate, dependable lab quality results with exceptional ease of use.
“Bringing MEMS technology into the medical device market is a real step change, unlocking the potential of advanced technology to create smaller, simpler-to-use devices for the growing healthcare diagnostics market,” Thurlow said. Based on Microvisk's estimates, the potential EU market for the device could reach US $350 million by 2023.
CoagMax® and its disposable strip are clearly distinct from competitors’ existing technology which, according to Microvisk, relies on older, more complex optical analysis or chemical reaction-based measurements.
Eliminates Need for Lab Testing
Taking the CoagMax® meter to the finger reduces patient time and inconvenience for clinic attendance, while eliminating the need for a laboratory. Requiring only 8 ul of blood – less than competing systems – also means less pain for patients, Microvisk said.
The disposable strip uses the standard international PT (Prothrombin Time) or INR (International Normalised Ratio) test, by using a drop of a patient’s whole blood from a finger prick. The sensors detect blood changes to a gel-like substance and evaluate whether viscosity is within acceptable range for the patient’s medication.
Beyond medical diagnostics, Microvisk’s technology platform has future potential for numerous fluid viscosity testing applications – with oils and foodstuffs being prime examples, according to industry analysts.
Source: Bionow.co.uk
Image Credit: Medgadget.com
Latest Articles
Stroke, blood clot, anti-coagulant, self-testing, viscosity
Microvisk Ltd.'s (North Wales, UK) first-of-its kind medical device – a disposable diagnostic strip based on a Micro-Electro-Mechanical System (MEMS) –...