ICU Management & Practice, ICU Volume 14 - Issue 2 - Summer 2014
Authors

Rajit K. Basu, MD
Center for Acute Care Nephrology
Cincinnati Children’s Hospital and Medical Center
University of Cincinnati, Department of Pediatrics
Cincinnati, Ohio, USA

Stuart L. Goldstein, MD
Center for Acute Care Nephrology
Cincinnati Children’s Hospital and Medical Center
University of Cincinnati, Department of Pediatrics
Cincinnati, Ohio, USA

John A. Kellum, MD, MCCM
Center for Critical Care Nephrology
Department of Critical CareMedicine
University of Pittsburgh
Pittsburgh, PA, USA
ICU Management Editorial Board Member
Acute kidney injury (AKI) is a significant problem in hospitalised patients, independently contributing to morbidity and increasing mortality. Prevention, risk assessment and supportive care, dependent on timely recognition of decreases in kidney function, are the mainstay of current treatment, as no singular therapy has proven effective at reducing AKI or improving associated negative outcomes. Additionally, although AKI severity-based care guidelines have been published, they have not yet been widely disseminated into clinical practice and AKI management continues to be highly variable. In this brief review, we discuss the potential benefits of an AKI care bundle to standardise supportive care measures. We suggest that incorporation of this bundle would help minimise practice variation, making possible the determination of effective disease- and patient-targeted therapy, and potentially lead to improved patient outcomes.
Introduction
Acute kidney injury (AKI) is an international epidemic and its incidence is increasing (Lameire et al. 2013; Rewa and Bagshaw 2014). Indeed, the incidence of AKI has grown at an alarming rate over the past 20 years and has been repeatedly shown to be associated with increased mortality, prolonged mechanical ventilation and longer hospital stay (Bellomo et al. 2012; Kellum et al. 2011; Hoste et al. 2010; Foretnberry et al. 2013). Increasing recognition of the negative impact of AKI has led to a paradigm shift – patients are not just dying with AKI, but from AKI (Hoste and Corte 2011). Since no therapy has yet proven to be effective to mitigate the deleterious effects associated with AKI, systematic risk assessment, preventive strategies and supportive care directed toward overall patient stability are the cornerstones of AKI management.
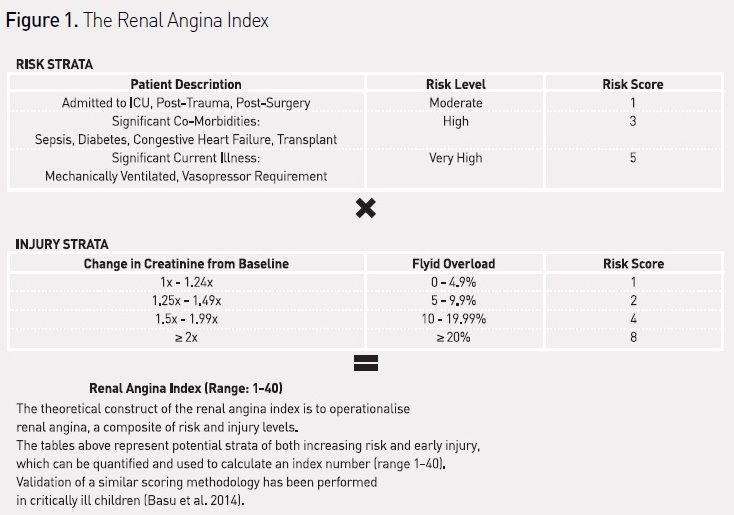
AKI prevention depends on systematic risk and disease recognition. To date, no widely accepted and implemented practice parameters exist to identify and triage at-risk patients even though many AKI risk factors are known. Further, while AKI in intensive care units (ICUs) most commonly occurs secondary to other systemic injury (sepsis, trauma, postsurgical), the risk is not binary. Epidemiologic data strongly suggest that in the ICU degrees of risk exist and it is not homogeneously ‘present or absent’. Stratifying the heterogeneity of patient risk is essential to implementation of a feasible AKI prediction strategy. Leveraging population analyses of risk factors in adults and children, the recently proposed renal angina methodology is the first attempt to use risk stratification to underscore patient risk and improve prediction of AKI (Goldstein and Chawla 2010).
Timely recognition of AKI has been challenged by limitations associated with the traditional parameters used for diagnosis. Changes in serum creatinine (SCr) and urine output (UOP) may lag behind injury, and are variable with regard to patient body habitus, gender, and age (Bagshaw and Gibney 2008). Thus, current AKI diagnostic strategy is often reactive to injury. By contrast, novel AKI biomarkers carry the potential to provide robust predictive ability in and may enable proactive therapy for incipient injury. Unfortunately, many of these biomarkers demonstrate inconsistent ability for AKI prediction in heterogeneous patient populations not stratified by risk (McCullough et al. 2013; Vanmassenhove et al. 2013; Basu et al. 2012). Although recent advances in biomarker development focusing specifically on discovery in heterogeneous populations appear to show more promise (Kashani et al. 2013; Cruz and Mehta 2014; Bihorac et al. 2014), patient selection for biomarker measurement remains essential. Critically ill patients are already a high-risk group. Identifying patients with increased risk outside the ICU appears to be more challenging.
Current AKI management is supportive in nature and generally directed at the pathophysiologic drivers of injury. Large randomised controlled trials (RCTs) studying more individualised therapy - diuretics for oliguria, anti-inflammatory medication, continuous renal replacement therapy (CRRT) for fluid overload, and ‘reno-selective’ vasodilators such as fenoldopam to augment renal perfusion have been attempted, but demonstrate inconsistent outcomes (Nigwekar and Waikar 2011; Palevsky 2009; Landoni et al. 2007; Scrascia et al. 2014). The dustbin of AKI trials is filled with these therapies, which demonstrate the ability to alter the course of a single perturbation (ie increase diuresis in oliguric patients), but are unable to affect the ultimate outcome of interest (eg AKI or decreased mortality rates). Consequently, no consistent and standard protocol is followed for AKI management and treatment continues to be directed towards global injury.
Risk Recognition to Trigger Management
To improve global recognition of risk factors and unify the approach to the diagnosis and care of patients with AKI, the Kidney Diseases Improving Global Outcomes (KDIGO) AKI collaborative published stage-based AKI practice guidelines (Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group 2012). These guidelines embody the efforts of the Agency for Healthcare Research and Quality (AHRQ) to standardise care for high-impact diseases by “reducing variations, improving outcomes, and reducing costs” (Agency for Healthcare Research and Quality 2013). There have been no published studies to date reporting on the implementation of these guidelines and outcomes from use.
The KDIGO AKI management guidelines are predicated on identification of AKI risk or injury. Unfortunately, unlike acute coronary syndrome, stroke, infection and many other acute illnesses, AKI does not carry easily identifiable physical signs to expedite identification of injury and initiation of management. For instance, while respiratory failure often presents with shortness of breath and myocardial infarction with chest pain, AKI does not typically present in a way that brings patients to medical attention (ie symptomatically). Simply put, AKI does not hurt (Goldstein and Chawla 2010). Despite limitations mentioned earlier, however, small changes in SCr and UOP below the level of injury as classified by consensus criteria have been associated with high rates of progression to severe AKI and worse hospital outcomes (Basu et al. 2012; Cruz et al. 2014; Coca et al. 2007; Nin et al. 2010; Zappitelli et al. 2009). So while chest pain in the correct context (angina pectoris) may be the herald sign for a heart attack, injury diagnosed by small changes in SCr or UOP in the correct context (renal angina) may be a harbinger of a kidney attack (Kellum et al. 2012). To operationalise this concept, the renal angina index (RAI) was derived (see Figure 1), combining AKI risk factors and sub-clinical injury (Basu et al. 2014). Context-driven biomarker testing for detection of early myocardial injury has dramatically improved the outcomes of patients suffering heart attacks as it triggers the initiation of heart attack protocols and stagebased management. Context-driven biomarker testing for detection of early AKI, using renal angina or other validated methodologies to identify high-risk patients, may now be possible, and lends itself to direct integration with management guidelines.
AKI Bundle – Targeting Risk, Injury, and Standardising Care
Improved outcomes in patients with AKI will rely on the development and incorporation of an automated AKI detection and management tool. Clinical decision support systems (CDSS) embedded within high functioning electronic health records (EHRs) are able to integrate patient information, laboratory data, and efficacy of available therapeutic options in real time. CDSS are currently being used to standardise management of several high impact disease processes to adhere to best practice guidelines (Cleveringa et al. 2007; Twiggs et al. 2004; Okelo et al. 2013).
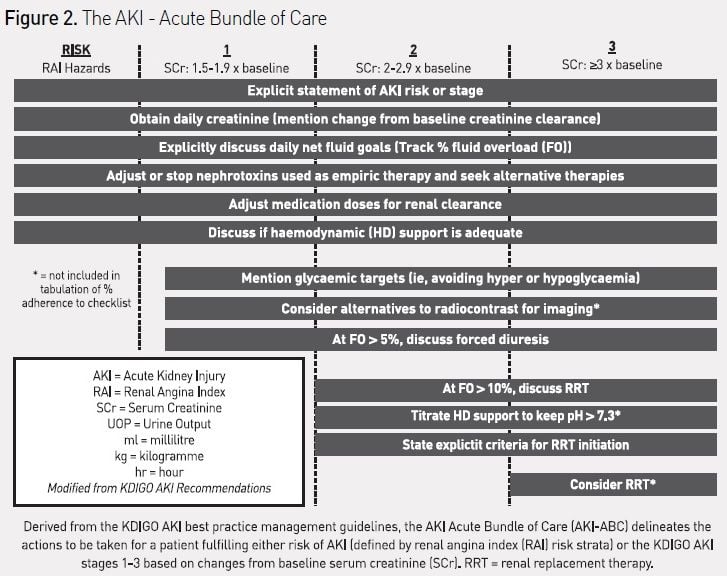
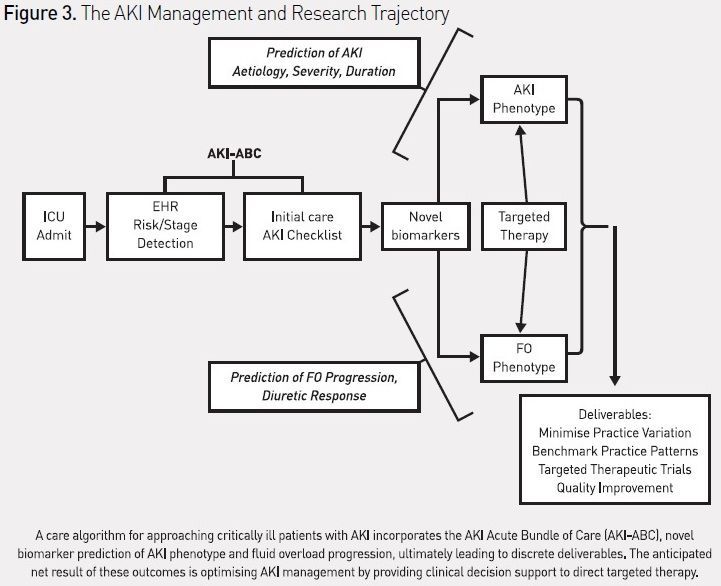
CDSS to guide AKI prediction and management in the ICU have not yet been described, but an ‘AKI Acute Bundle of Care’ (AKI-ABC), incorporating risk stratification, renal angina, and AKI-stage-based management parameters can be designed and potentially incorporated into an EHR (see Figure 2). The integration of this bundle into patient workflow would theoretically occur during the initial stabilisation phase of admission and twice daily thereafter. The bundle explicitly incorporates the stages of AKI, including risk of AKI. The bundle focuses the attention of care providers on routine practices, which can both prevent AKI from occurring (such as discontinuing nephrotoxic agents or using alternatives to intravenous contrast) and mitigate the effects of AKI (limit the extent of fluid overload (FO)). Detection of vital patient elements and calculation of risk could be programmed using natural language processing or other advanced biomedical informatics technique (Campas et al. 2013; Byrd et al. 2013). Additionally, the bundle attempts to standardise the approach to initiation of renal replacement therapy (RRT), for which there currently is no consistent and universally accepted algorithm. Similar to the reported effect of an EHR-based trigger tool for detection of nephrotoxic-associated AKI triggering increased systematic kidney function surveillance, a reduction in the severity or duration of AKI, or the prevalence of AKI, would be anticipated with adherence to this bundle (Goldstein et al. 2013). The use of the bundle in critically ill patients would also standardise management and minimise practice variation, making it possible to systematically investigate new diagnostic strategies (e.g. combining biomarkers or attempting to phenotype AKI by pathophysiology) or targeted therapeutic interventions (Endre et al. 2013; McCullough et al. 2013). The incorporation of this checklist into the AKI research trajectory, studying EHR use, novel biomarker diagnosis, and creation of AKI-disease phenotypes (e.g. fluid overload), could then be used to benchmark practice patterns against the outcome of disease (see Figure 3). The development of quality improvement benchmarks for internal and external reporting would logically follow. For a global disease, the adoption of such standards would make side-by-side comparisons of AKI epidemiologic patterns and outcomes possible across not only institutions, but different socioeconomic and geographic areas.
Conclusion
The epidemic of AKI demands action; increasing recognition of the negative impact of kidney attacks will drive the impetus for a more logical, consistent, and algorithmic approach to the disease process. Use of the EHR to integrate risk stratification, disease severity and an initial AKI management bundle would be an example of a highly useful clinical decision support tool. Standardisation of the approach to AKI would minimise the variability of practice and potentially allow disease and patient-specific targeted therapeutic trials to be developed. This methodology may be the path forward.
For full references, please email [email protected], visit the website at www.icu-management.orgor use the QR code at the top of the article.



















