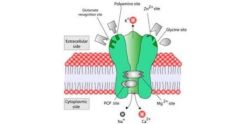
According to findings from a Phase 3 clinical trial, there may be an additional therapeutic option for seizure control in patients suffering from epilepsy.
The findings are to be unveiled at the 68th Annual Meeting of the American Epilepsy Society. The double-blind, randomised, placebo controlled study was conducted by researchers from the Mid-Atlantic Epilepsy and Sleep Center and other participating medical centres.
The study evaluated the efficacy and safety of the drug brivaracetam, an analog of the commonly used anti-epileptic drug (AED) levetiracetam, in adults with poorly controlled partial onset seizures.
“This first presentation of primary study results from the latest Phase 3 brivaracetam study is highly anticipated in the epilepsy community. The two primary outcomes in this study evaluating adjunctive brivaracetam in the treatment of partial-onset seizures in adults with epilepsy were statistically significant and clinically relevant,” said Dr. Pavel Klein, Director of the Mid-Atlantic Epilepsy and Sleep Center.
The study participants were 764 adults who were taking one or two AEDs concurrently. Over 80 percent of the study patients had already failed at least two epilepsy medications, while 47 percent had failed five or more. The patients were given fixed doses of 100mg/day or 200 mg/day brivaracetam as an adjunctive treatment.
After a baseline period of eight weeks, the patients continued for another 12 weeks of brivaracetam treatment, after which they became eligible for long-term follow-up to monitor the drug’s efficacy and safety.
The study showed that compared to placebo, there was a clinically relevant reduction in the frequency of partial onset seizures during a 28-day period in patients taking brivaracetam. A dose of 100 mg brivaracetam resulted in a 22.8 percent decline in seizure frequency over a 28-day period, while 200 mg brivaracetam reduced seizures by 23.2 percent. The study authors report that the findings were consistent regardless of prior levetiracetam exposure.
23 patients treated with brivaracetam experienced complete freedom from all seizure types, as compared to only two patients in the placebo group.
No major side effects were observed with the drug and the authors report that overall the drug was well-tolerated. The most common adverse effects were dizziness, fatigue, headache and sleepiness, which occurred in 7-19 percent of patients taking 100 mg and 8-16 percent of patients taking 200 mg brivaracetam, compared with 3-8 percent of patients in the placebo group.
The authors conclude that daily doses of 100 mg and 200 mg brivaracetam are well-tolerated and may help reduce seizure frequency when administered as adjunctive therapy in adult epilepsy patients with partial onset seizures.
Source: Newswise
Image Credit: Wikimedia Commons