ICU Management & Practice, Volume 19 - Issue 2, 2019
A case from a specialised weaning centre
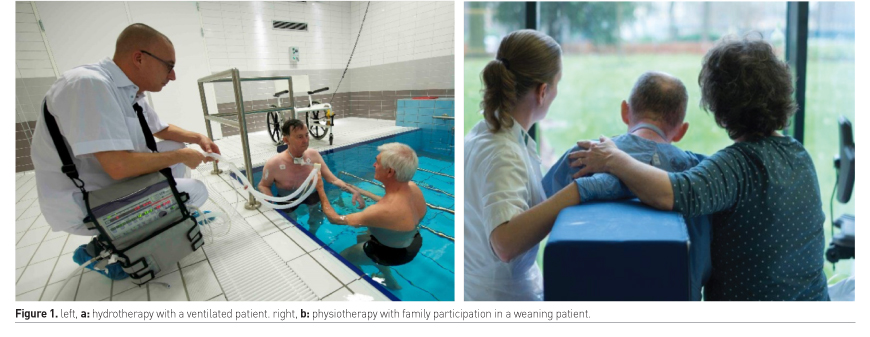
Case
Airway and lung dysfunction
Brain dysfunction
Cardiac dysfunction
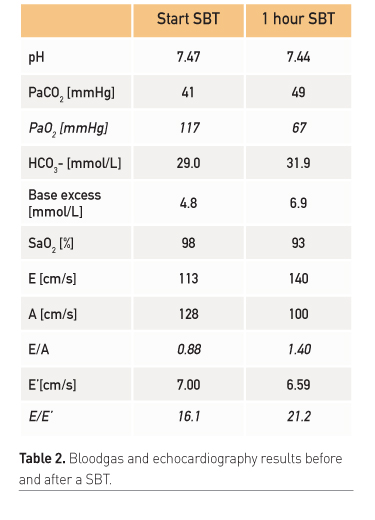
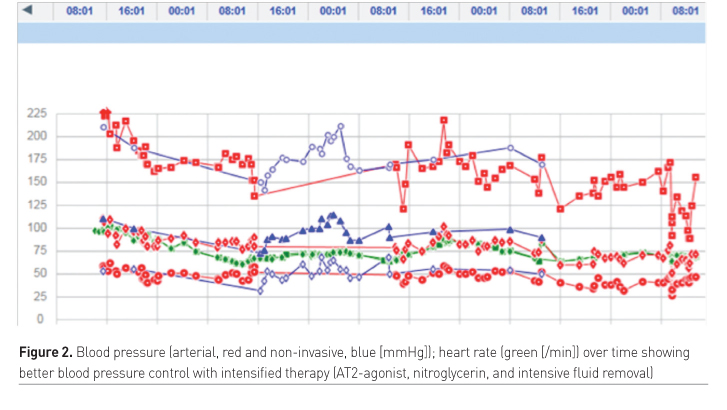
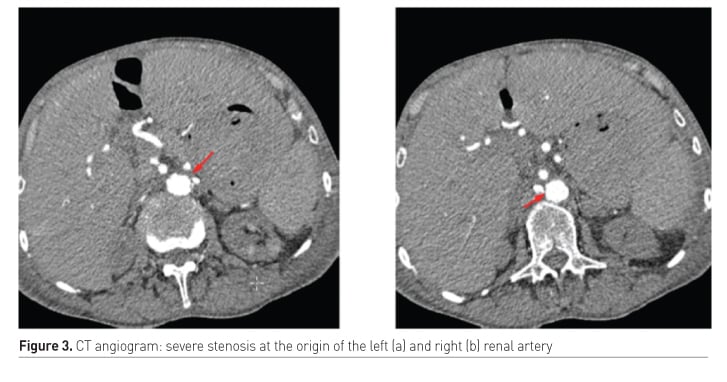
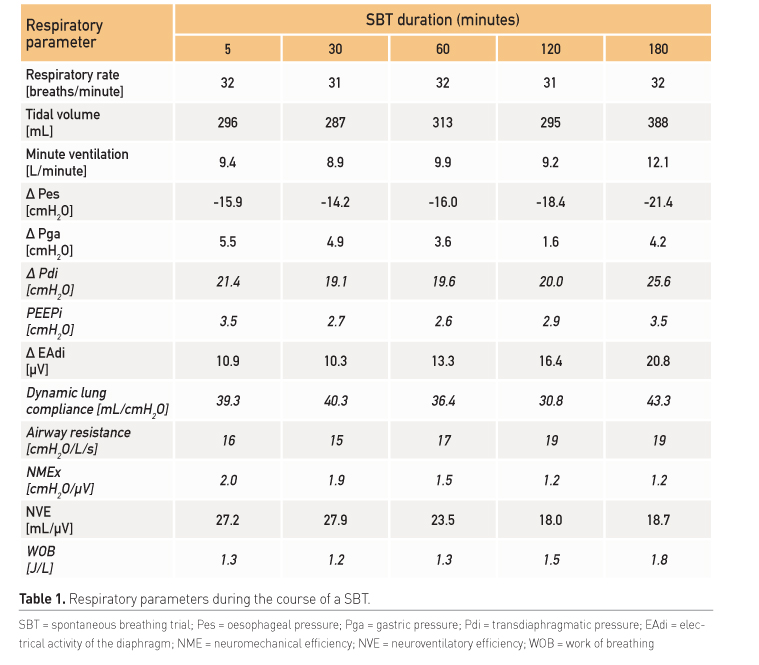
Diaphragm/respiratory muscle function
Endocrine and metabolic dysfunction
Feeding and dysphagia
Conclusion
- CHF with preserved ejection fraction (diastolic dysfunction)
- Acute-on-chronic kidney failure
- Difficult to treat hypertension due to renal artery stenosis
- o Leading altogether to peripheral and lung oedema
- No angiographic/surgical intervention due to severe atherosclerosis with increased risk of complications
- Intensive fluid removal with haemodialysis (total negative fluid balance 9.6 L)
- Nitroglycerin during SBT (systolic blood pressure < 150 mmHg) to prevent CHF due to increased afterload
- Treatment of hypertension with AT2 antagonist
- Our patient could be successfully weaned from the ventilator and extubated within 4 days.
Key points
- Factors increasing the work of breathing and thereby contributing to weaning failure, are increased airway resistance, decreased lung or chest wall compliance and impaired gas exchange.
- Brain dysfunction is associated with a higher risk of failed extubation and anxiety, sleep disturbances and depression may interfere with successful weaning.
- During mechanical ventilation respiratory muscle dysfunction rapidly develops and is associated with difficult weaning, but not with peripheral muscle weakness.
- Adrenal insufficiency and hypothyroidism have been described as possible reasons for weaning failure accompanied by successful treatments.
- Malnutrition frequently occurs in critically ill patients and is associated with higher mortality and reduced muscle mass contributing to difficult weaning.
- An individualised structural evaluation of weaning failure helps to find the underlying causes of weaning failure and to prescribe an individualised treatment plan.
References:
American Thoracic Society/European Respiratory S (2002) ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med, 166(4): 518-624.
Datta D. and P Scalise (2004) Hypothyroidism and failure to wean in patients receiving prolonged mechanical ventilation at a regional weaning center. Chest, 126(4): 1307-1312.
Doorduin J, Roesthuis LH, Jansen D, van der Hoeven JG, van Hees HWH, Heunks LMA. (2018) Respiratory muscle effort during expiration in successful and failed weaning from mechanical ventilation. Anesthesiology, 129(3): 490-501.
Doorduin J, van Hees HWH, van der Hoeven JG, Heunks LMA. (2013) Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med, 187(1): 20-27.
Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A (2017) Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med,195(1): 57-66.
Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X (2014) Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med, 42(8): 1882-1889.
Dres M, Teboul JL, Monnet X (2014) Weaning the cardiac patient from mechanical ventilation." Curr Opin Crit Care 20(5): 493-498.
Felten-Barentsz KM, Haans AJ, Slutsky AS, Heunks LM, van der Hoeven JG (2015) Feasibility and safety of hydrotherapy in critically ill ventilated patients. Am J Respir Crit Care Med 191(4): 476-477.
Frenzel T, Roesthuis LH, van den Boogard M, Zegers M; van der Hoeven JG (2018) Preliminary results of the first dedicated Dutch ICU for complex weaning from mechanical ventilation. 6th European Conference on Weaning and Rehabilitation in Critically Ill Patients. Leuven.
Geense W, Zegers M, Vermeulen H, van den Boogaard M, van der Hoeven J. (2017) "MONITOR-IC study, a mixed methods prospective multicentre controlled cohort study assessing 5-year outcomes of ICU survivors and related healthcare costs: a study protocol. BMJ Open 7(11): e018006.






















