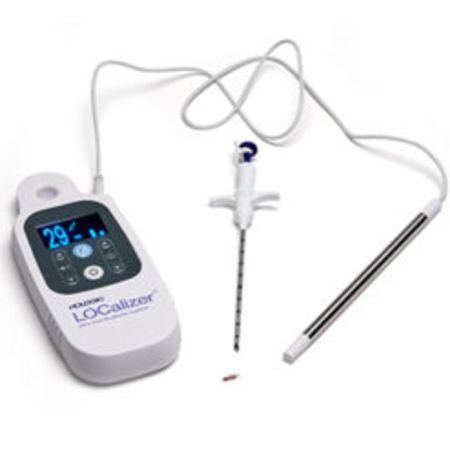
Wire-free lesion localization tags can now be implanted for more than 30 days, creating greater flexibility for providers and patients during breast-conserving surgeries
Hologic today announced the extension of the CE Mark for its LOCalizer™ radiofrequency identification (RFID) tag for long-term placement. The tag can now be implanted more than 30 days prior to a breast-conserving surgery, providing even greater flexibility and convenience to patients and providers.
The LOCalizer wire-free guidance system is a non-radioactive, radiofrequency localization system designed for precise marking and targeting of lesions in breast-conserving surgery. It was designed to replace the traditional wire-guided localization method, which requires placement of a wire on the day of surgery. With this new tag, placement can be done weeks or months prior to surgery. For patients, this means they can arrive closer to their surgery time and experience fewer interventional procedures.
Following its earlier placement, the miniature implantable tag can be detected on the day of surgery by a portable, handheld reader that indicates the location and distance in mm to the lesion, enabling the surgeon to pinpoint the correct area of breast tissue for removal. Each tag also features a unique identification number that is displayed on the reader. This improved workflow is designed to help reduce scheduling and logistical hurdles for care teams, and aims to deliver added convenience for an enhanced patient experience.
“The extension of LOCalizer’s CE Mark is yet another reflection of our commitment to continue to seek innovative solutions that create a more positive experience for both patients and physicians,” said Jan Verstreken, Hologic's Regional President, EMEA, Canada & LatAm. “It also represents another way we are working to improve breast health throughout the entire patient journey, including for complex breast conserving surgeries.”
The LOCalizer system is part of a growing portfolio of breast surgery and pathology solutions resulting from Hologic’s recent acquisition of Faxitron. For more information about the LOCalizer system, visit https://hologicbreastsurgery.com/en/portfolio/localizer-wire-free-guidance-system/ The LOCalizer system is manufactured by Health Beacons, Inc. and is exclusively distributed by Hologic.
Latest Articles
Hologic, CE Mark, Jan Verstreken, LOCalizer, breast surgery, RFID, breast-conserving surgeries, radiofrequency identification, pathology solutions
Wire-free lesion localization tags can now be implanted for more than 30 days, creating greater flexibility for providers and patients during breast-conserving surgeries