In a study published online by the Journal of Magnetic Resonance Imaging, researchers have found that the diagnostic performance of MultiHance ® (gadobenate dimeglumine is equivalent to that of a double dose of gadopentetate dimeglumins in MR angiography.
The results of the Peripheral VALUE study show that a single, standard dose of MultiHance ® (active ingredient: gadobenate dimeglumine), provides at least equivalent contrast enhancement and diagnostic performance to a double dose of gadopentetate dimeglumine (Gd-DTPA) in Magnetic Resonance Angiography (MRA) of the peripheral arteries.
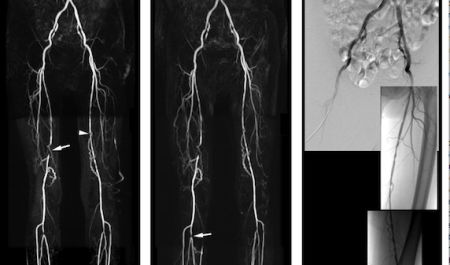
As part of the clinical development of MultiHance ® in MRA in China, a multicentre, intra-patient, crossover comparison was conducted in 68 patients with peripheral arterial occlusive disease (PAOD). All patients underwent two separate contrast-enhanced MRA exams, one with 0.1 mmoL/kg MultiHance®, the approved dose for this indication in Europe and the U.S., and one with a double dose (0.2mmoL/kg) of gadopentetate dimeglumine (Gd-DTPA). The imaging protocol was kept identical for all exams, apart from the contrast agent used. Three independent and experienced radiologists, unaffiliated with the study sites and blinded to patient information and contrast agent, separately and independently assessed all MRA images from all patients in terms of contrast enhancement, vessel anatomical delineation and disease detection. The diagnostic performance (sensitivity, specificity, accuracy, predictive values) of the two MR exams in the detection and assessment of extent of steno-occlusive disease was measured in 53 patients who had also undergone the gold standard procedure, digital subtraction angiography. The results of this study showed a trend of superiority for MultiHance ®, though differences were not statistically significant.
"The results of this study as well as the superiority shown by MultiHance® in a previous multicentre study when tested at the same dose of the comparator, can be ascribed to its relaxivity, roughly two-fold higher than that of Gd-DTPA and other agents", said Dr. Alberto Spinazzi, Head of Global Medical and Regulatory Affairs at Bracco Imaging. "Notion that MultiHance ® provides superior diagnostic performance than lower relaxivity agents decreases the need and the likelihood of using doses higher than the approved 0.1 mmol/kg dose in peripheral MRA."
In Europe, MultiHance ® is approved not only for MRA, but also for other applications, including MRI of the liver and MRI of the CNS in adults and paediatric patients. "When considering the approved MultiHance® indications, which account for the vast majority of all contrast enhanced MRI procedures performed in Europe, radiologists can count on compelling evidence and clinical data demonstrating the high diagnostic value of our product", noted Micol Fornaroli, Chief Strategy Officer and Head of Global Marketing at Bracco Imaging.
Said Fulvio Renoldi Bracco, Head of Global Business Unit Imaging at Bracco, "The unique characteristics of MultiHance® and its demonstrated clinical value for MRA are well known to European radiologists for several years now. In August 2012 MultiHance ® was also approved for MRA in the United States, where it is now indicated for use in patients with known or suspected renal or aorto-ilio-femoral occlusive vascular disease; this provdes that confidence that also the Food and Drug Administration has in our product."
Latest Articles
In a study published online by the Journal of Magnetic Resonance Imaging, researchers have found that the diagnostic performance of MultiHance ® (gadobena...