System addresses unmet need for continuous glucose monitoring in the ICU, a potential market in excess
of $5 billion
GlySure Limited has announced that it has secured the CE Mark
for the world’s first and only Continuous Intravascular Glucose Monitoring System (CIGMS). The initial use
of the GlySure™ CIGMS is to enhance blood glucose management among adult cardiac surgery patients in
the Intensive Care Unit (ICU). The need for enhanced glucose management exists within all critical care
settings.
1,2 GlySure is currently conducting a UK-based multicentre trial designed to enable use of the
GlySure CIGMS across all adult Intensive Care patients.
In critically ill patients, poorly controlled blood sugar levels can lead to increased mortality, morbidity,
lengths of stay in the ICU and costs to healthcare providers.3 This CE Mark clears the path for GlySure to
market its GlySure CIGMS in Europe. The Company will first bring this solution to leading critical care
centres in the UK, Benelux and Germany, followed by launches in additional European countries and other
markets where CE Mark is recognised.
“For over a decade, the clinical community has been seeking a way to tightly control glucose levels in
critically ill patients for both improved outcomes and reduced costs,” said Dr. Krishna Prasad, MD FRCA
CCST, Consultant Anaesthesiologist at Care Hospitals, Nampally in Hyderabad, India, who was the
Principal Investigator of the CIGMS CE marking trial. “GlySure’s technology enables them to do this safely
with accuracy, reliability and efficiency to support the implementation of improved Glycaemic Control
protocols.”
“We are pleased to bring to market the first practical solution to address this significant unmet medical
need,” said Roger Moody, Chief Operating Officer for GlySure. “Our first customers will lead the way in how
glucose management is best practised, enabling improved patient outcomes and reduced healthcare costs.
Continuous glucose monitoring in critical care has been a vexing medical challenge. GlySure’s game
changing technology and elegant CIGMS solution will be the first in this market to deliver clinical value to
patients and savings to healthcare providers.”
GlySure received its CE Mark following a review of its submission that included the results of its multicentre
clinical trial that demonstrated the system’s ability to provide accurate continuous blood glucose monitoring
in patients throughout their length of ICU stay. The GlySure CIGMS met the primary safety and efficacy
endpoints and demonstrated a consistently high level of accuracy compared to a “gold standard”
intermittent glucose analyser.
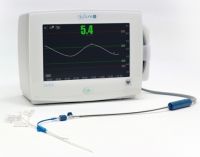
The GlySure CIGMS comprises of three main parts: a monitor, a disposable fibre optic sensor and a
disposable 5 lumen central venous catheter (CVC), similar to that typically used in the ICU. The GlySure
sensor includes a highly selective proprietary chemistry that provides the first commercially available
glucose testing system that can accurately measure intravascular glucose levels every fifteen seconds.
This breakthrough provides physicians with continual feedback that helps avoid dangerous fluctuations in
ICU patient glucose levels.
GlySure intends to seek clearance from the U.S. Food and Drug Administration (FDA) for the GlySure
CIGMS and is currently finalising the design of the clinical trial to support its submission. The Company has
recently appointed Albert Leung, MD PhD as its Chief Medical Officer who will lead these clinical and
regulatory efforts. Dr. Leung is an endocrinologist with 20 years of clinical practice and industry experience,
and had previously worked at Merck and Johnson & Johnson to bring new products to market by leading
their clinical development programs.
GlySure estimates the global market opportunity for continuous glucose monitoring in the ICU is greater
than $5 billion. This calculation takes into consideration the total number of patients in a critical care setting
worldwide.
References:
1. Van den Berghe G, et al. Intensive Insulin Therapy in Critically Ill Patients. N Engl J Med. 345,
1359–1367 (2001).
2. Krinsley JS, Preiser J. Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated
with increased survival in non-diabetic critically ill adults. Critical Care. 19, 179 (2015).
3. Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients.
Chest. 129(3), 644-650 (2006).
4. Van den Berghe G, et al. Analysis of healthcare resource utilization with intensive insulin therapy in
critically ill patients. Crit Care Med. 34(3), 612-6 (2006).
5. The NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N
Engl J Med. 367, 1108-1118 (2012).
Source and image credit: GlySure
Latest Articles
GlySure CIGMS, CE Mark, CVC, Intensive Care Unit
In critically ill patients, poorly controlled blood sugar levels can lead to increased mortality, morbidity, lengths of stay in the ICU and costs to healthcare providers.3 This CE Mark clears the path for GlySure to market its GlySure CIGMS in Europe