New Biomarkers Improve Risk Assessment Of Acute Kidney Injury
Lisbon, Portugal – October 15, 2012 - Astute Medical, Inc. announced it has completed CE mark certification for its first product, the NEPHROCHECKTM Test, and is commencing sales. The NEPHROCHECK™ Test utilizes two newly validated urinary biomarkers for risk assessment of acute kidney injury (AKI) in critically ill patients. The NEPHROCHECK™ Test is not available for sale in the United States.
AKI is a complex and increasingly prevalent condition associated with high mortality in critically ill patients. Although it occurs quickly (over the course of hours), there is commonly a challenge in risk assessment due to the inadequate tools currently available to physicians. Failure to recognize and manage AKI in the early stages can lead to devastating outcomes for patients and increased costs to the healthcare system.
“Acute kidney injury is common, with a prevalence similar to heart attack, and can impose significant cost in hospitals,” said Chris Hibberd, Astute Medical chief executive officer. “Our research suggests that intensivists and nephrologists are eager for new tools that can be used in combination with existing guidelines to advance both the management of patients, and the possibilities of their recovery.”
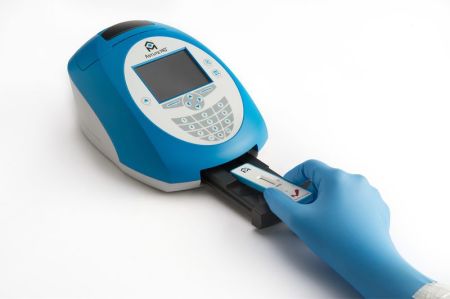
The NEPHROCHECK™ Test provides risk assessment of critically ill patients who may develop acute kidney injury. By producing results in approximately 20 minutes, the test aids clinicians in managing critically ill patients at risk of or affected by AKI. The test is run using a human urine sample, and multiple levels of controls ensure reliable and accurate test results.
Both large and small hospital laboratories can easily implement the new risk assessment tool. The immunoassay test is performed using a small, single-use NEPHROCHECK™ Test cartridge and a compact platform, the ASTUTE140™ Meter. This easy-to-use platform converts the fluorescent signals for each specific novel biomarker into concentrations, combining them into a single numerical test result.
Astute Medical has completed CE Mark certification for the ASTUTE140™ Meter. The ASTUTE140™ Meter is not available for sale in the United States.
Source:Astute Medical
www.astutemedical.com