ICU Management & Practice, Volume 16 - Issue 2, 2016
A Public Health Threat That Should Be Routinely Included Within Care Quality and Patient Safety Programmes
This paper provides an overview of the evidence confirming that CDI independently increases mortality risk in hospitalised patients, and argues for system-wide implementation of specific actions, including care bundles for management (not only infection control) and mandatory surveillance, to improve the quality of CDI care, and thereby reduce morbidity and mortality, in alignment with European policy initiatives.
Patient safety, defined as the freedom for patients from unnecessary or potential harm associated with healthcare (Council of the European Union 2009), is a central component of healthcare quality. Healthcare-associated infections (HAIs) are a leading threat to patient safety. On average, 6% of patients in European acute care hospitals have at least one HAI (European Centre for Disease Prevention and Control [ECDC] 2013). Annually these infections are estimated to cause 37,000 deaths and to incur costs of over €5.5 billion (Committee on the Environment, Public Health and Food Safety 2013). In 2009 the European Council recommended that Member States implement various measures to improve patient safety and HAI prevention and control in particular (Council of the European Union 2009). The implementation of these measures has been supported by the European Union Network for Patient Safety and Quality of Care Joint Action (pasq.eu). Progress has been made (European Commission 2012; European Commission 2014), and there is a raised level of awareness about patient safety at the political level. Nevertheless, the European Parliament has urged the Commission and Member States to step up their efforts and to place the issue near the top of the political agenda (Committee on the Environment, Public Health and Food Safety 2013), and the European Council has invited the Commission to continue supporting improvements in patient safety and HAIs and the Member States to intensify their efforts in these areas (Council of the European Union 2014). Specific considerations include the setting of national targets, resourcing, education and training of healthcare professionals, the promotion of good practice, information provision to patients, disease surveillance and research support.
Although figures vary between countries, Clostridium difficile (C. difficile) infection (CDI) accounts for almost 4% of all HAIs in Europe, and C. difficile accounts for 5.4% of isolated pathogens, being the eighth most common (ECDC 2013). CDI is increasingly common in many countries and the estimated annual number of 124,000 cases across Europe (ECDC 2013) is likely to be an underestimate owing to under-diagnosis (ECDC 2013; Davies et al. 2014). In the United States, a multistate prevalence survey concluded that C. difficile was the most commonly reported pathogen, causing 12% of all HAIs (Magill et al. 2014). The Centers for Disease Control and Prevention (CDC) estimates that 453,000 CDI cases occur annually in the U.S. (Lessa et al. 2015) and categorises CDI in the highest priority category of antimicrobial resistance threats (CDC 2013). In a systematic assessment of antimicrobial resistant threats based on 10 criteria, the public health agency of Canada also defined C. difficile as the second most important national priority (Garner et al. 2015). CDI typically adds approximately €4000–14,000 to inpatient costs, mainly as a result of extended hospitalisation (Weigand et al. 2012; Asensio et al. 2015; Heimann et al. 2014).
CDI has been the focus of comprehensive and effective national-level control and surveillance interventions in some countries (e.g. United Kingdom), but remains under-recognised as a public health threat in many others. Indeed a discrepancy exists whereby, in many countries, efforts have focussed on multi-drug resistant Gram-negative pathogens and methicillinresistant Staphylococcus aureus, while CDI presents a greater threat to public health (CDC 2013).
CDI and Mortality
Patients who acquire CDI in healthcare facilities are at high risk of death. Rates of all-cause 30-day mortality are estimated at 3–30% among all patients (Weigand et al. 2012; Wenisch et al. 2012; Hensgens et al. 2013; Schmid et al. 2013; Planche et al. 2013), and reach 40% among patients who undergo emergency surgery for fulminant CDI (Bhangu et al. 2012). However, as CDI is most common among patients who are elderly and suffering from severe underlying illnesses (Bauer et al. 2011), specific studies were necessary to confirm its independent contribution to the risk of death.
A European-wide epidemiological study conducted in 2008 found that CDI caused or contributed to death in 9% of 455 infected patients within 3 months of diagnosis. Expressed differently, CDI caused or contributed to 40% of all deaths occurring within 3 months (Bauer et al. 2011). More recently a large prospective cohort study in nine hospitals in the Netherlands showed that even in an endemic situation (i.e. in the absence of outbreaks), CDI independently increased the risk of 30-day mortality by 2.5-fold (95% confidence intervals [CI] 1.4–4.3), as compared with patients without diarrhoea, after adjustment for age, sex and underlying diseases (Fig. 1) (Hensgens et al. 2013). Similarly a single-centre, prospective cohort study in Austria found a relative risk for pre-discharge death among patients with CDI of 2.74 (95% CI 1.82–4.10; p<0.0001), as compared with patients without CDI (Wenisch et al. 2012).
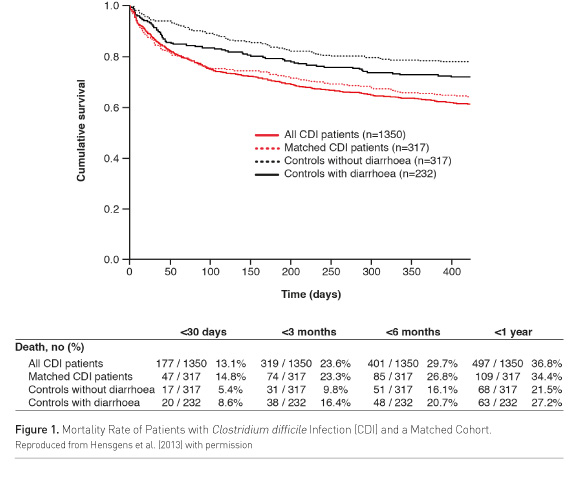
In the Netherlands, patients with CDI were more likely to die within 30 days (hazard ratio 1.6; 95% CI 0.9–2.8) than were controls who had diarrhoea but a negative test for C. difficile toxins—indicating that CDI, rather than diarrhoea per se, increased the mortality risk (Hensgens et al. 2013). Similarly, a prospective multicentre study of over 6500 CDI episodes in the UK showed that patients whose unformed faecal samples tested positive for the C. difficile toxins had a higher 30-day mortality (after multivariate analysis to account for In the Netherlands, patients with CDI were more likely to die within 30 days (hazard ratio 1.6; 95% CI 0.9–2.8) than were controls who had diarrhoea but a negative test for C. difficile toxins—indicating that CDI, rather than diarrhoea per se, increased the mortality risk (Hensgens et al. 2013). Similarly, a prospective multicentre study of over 6500 CDI episodes in the UK showed that patients whose unformed faecal samples tested positive for the C. difficile toxins had a higher 30-day mortality (after multivariate analysis to account for confounding factors) than those who were negative both for C. difficile toxins and cultures (odds ratio 1.61; 95% CI 1.12–2.31; p=0.01) (Planche et al. 2013).
Is CDI any worse than other types of infective diarrhoea? Researchers in Austria found that patients with CDI were twice as likely to die while in hospital or within 30 days than patients with other types of infective diarrhea (caused by norovirus campylobacter, adenovirus, salmonella or rotavirus) after adjustment for age, comorbidities and other infections (Schmid et al. 2013). From a public health perspective, CDI is estimated to cause 70% of all deaths due to gastroenteritis in the United States—ten times more than the next leading gastroenteritis pathogen, norovirus (Hall et al. 2012). According to recent CDC figures, CDI is associated with 29,300 deaths annually in the US (Lessa et al. 2015).
Mortality from CDI has been affected by changes in the epidemiology of the infection. The worldwide emergence of type 027 as a more virulent type with increased morbidity and mortality has been recognised since 2003 (Kuijper et al. 2006). Since then other highly virulent types have also been observed. These include types 018, 056 and 078 in Europe (Bauer et al. 2011; Walker et al. 2013), the last reportedly associated with higher mortality rates than type 027, and type 244 in Australia (Lim et al. 2014).
Reducing CDI-Related Mortality
CDI can affect patients in all medical specialties. Efforts to reduce its impact on patients and health systems must involve all healthcare professionals, hospital/health institution managers, ancillary staff (including janitors) and patients themselves, not merely microbiologists and infection specialists. Approaches to reducing CDI-related mortality include strategic, health system-wide measures to optimize diagnosis, therapy and infection control according to recommendations (Crobach et al. 2009; Debast et al. 2014; Vonberg et al. 2008; Dubberke et al. 2014).
Diagnosis
Prompt CDI diagnosis can shorten the time to treatment and reduce empirical therapy, and enables infection control measures to be implemented (Barbut et al. 2014). A delay in CDI therapy owing to a failure of diagnosis may increase the risk of mortality, especially in patients with severe CDI (Berman et al. 2008). C. difficile testing policies and practices have improved in recent years, and yet almost a quarter of CDI episodes still go undiagnosed (Davies et al. 2014). Education directed to all healthcare staff regarding the appropriate management of patients with potentially infectious diarrhoea is therefore central to efforts to prevent and control CDI. For example, all staff members need to know that if they have a patient with potentially infectious diarrhoea, they should send a sample for laboratory testing, to include C. difficile tests, as soon as possible and manage the patient with appropriate infection control measures, e.g. contact precautions. Educating patients with regard to the possible implications of CDI and the need for them to report diarrhoea while in hospital, is also important.
Therapy
Various experimental treatments for CDI are in development, ranging from new antibiotics to humanised anti-toxin monoclonal antibodies and faecal transplantations (Oldfield et al. 2014). Clinical trials have not been designed or powered to assess the benefit of therapy for CDI on mortality. In any event, regulatory Phase III trials of new agents would not be the best tools to assess ‘real-world’ mortality benefits owing to the exclusion criteria employed. Retrospective observational evidence suggests that patients with CDI are more likely to die within 30 days if treatment fails to improve symptoms within 10 days (Kim et al. 2013). The use of oral vancomycin rather than metronidazole for severe CDI, as recommended (Debast et al. 2014), provides clinical benefit, although a numerical benefit on mortality did not reach statistical significance (Le et al. 2012). Recently, a large retrospective cohort study in the USA reported that patients with recurrent CDI had a 33% higher risk of death at 180 days compared with patients who had a primary CDI episode but no recurrence (hazard ratio 1.33; 95% CI 1.12–1.58; p=0.001) (Olsen et al. 2015). A prospective UK study also found that patients with recurrent CDI were more likely to die within 1 year from the first episode (9/55; 16.4%) than were patients with non-recurrent infection (1/184; 0.5%; p<0.001) (Taori et al. 2013).
Data on real-world treatment patterns in Europe are scarce. However, evidence from the United States suggests that treatment and adherence to guidelines are suboptimal, especially in severe, complicated or recurrent CDI (Curtin et al. 2013). Although surgery can be life-saving in severe or complicated CDI (Bhangu et al. 2012), this depends critically on the indication, type and timing and further research is required.
Infection Control and Prevention
Adherence to recommended measures for the control and prevention of CDI (Vonberg et al. 2008; Dubberke et al. 2014) is essential to reduce the burden of infection. While these measures are common to other HAIs, CDI-specific procedures, such as careful handwashing using soap and water (rather than alcohol hand rubs), systematic glove wearing, sporicidal environmental decontamination and antibiotic stewardship, require specific education and resourcing. Importantly, antimicrobial stewardship programmes that reduce the use of high-risk antibiotics may contribute to a reduction in the rate of CDI cases. According to a recent meta-analysis of interventional studies, restrictive antibiotic stewardship policies halved the risk of CDI and had particular effect in elderly care settings (Feazel et al. 2014). Evidence suggests that gastric acid suppressants also increase the risk of CDI and should be considered in prevention strategies (Kwok et al. 2014).
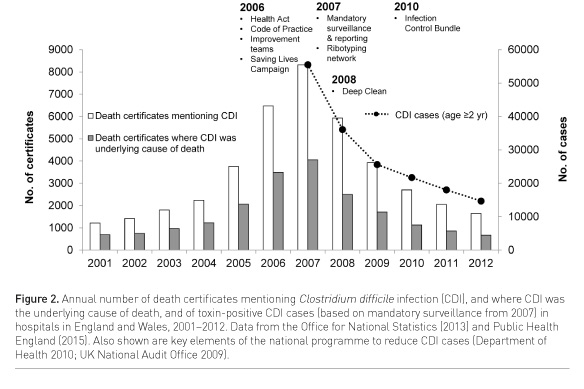
Infection control and prevention bundles can reduce the incidence of CDI and control hospital outbreaks (Muto et al. 2007; Abbett et al. 2009). A bundle is a small set of evidence-based interventions for a defined patient segment/population and care setting that, when implemented together, will result in significantly better outcomes than when implemented individually (Resar et al. 2012). At a national level, a ‘high impact intervention’ bundle was implemented across the UK to prevent CDI and to control outbreaks caused by the type 027 strain (Department of Health 2010). This bundle (available online) combined elements of prudent antibiotic prescribing, correct hand hygiene, environmental contamination, personal protective equipment (e.g. gloves and aprons), and isolation or cohort nursing. It was introduced in 2010 as part of a national programme of actions (beginning in 2006) that include legislation on infection control in hospitals, hand hygiene campaigns, improvement teams, mandatory surveillance and reporting, diagnosis and testing guidelines and a network of ribotyping laboratories (UK National Audit Office 2009). The annual number of CDI cases in England has decreased by approximately 80% since mandatory reporting was introduced in 2007/8, with a corresponding fall in the number of deaths (Fig. 2) (Office for National Statistics 2012; Public Health England 2015).
Care Quality Bundle for CDI Management
While bundles exist for CDI prevention and infection control, there is a limited interventional evidence base for a corresponding bundle designed to improve CDI management in order to avoid complications, recurrence and mortality. Nevertheless, an intervention programme was recently reported to have reduced delays in the initiation of CDI therapy and increased adherence to practice guidelines (Jury et al. 2013). This programme comprised physician education on CDI diagnostic testing and treatment recommendations and a CDI order menu (including diagnostic and therapeutic recommendations) implemented in the electronic medical record system. A CDI stewardship team (comprising a nurse practitioner and an infectious-diseases physician, with intermittent assistance from an infectious-diseases fellow and an infectious-diseases pharmacist) was notified by the microbiology laboratory of all positive C. difficile test results. This team reviewed electronic medical records and either provided initial treatment recommendations or, if treatment was ordered, provided feedback to physicians if management was not in accordance with guidelines. Finally, medical records were reviewed for a sample of patients prescribed empirical CDI therapy, and feedback was provided to physicians on adherence to the relevant recommendation on empirical therapy. Other workers have described a severity-based treatment policy that increased vancomycin use (according to guidelines) and reduced the rate of refractory disease in patients with severe CDI (Jardin et al. 2013). Such a bundle is not a ‘magic bullet’, but is part of a proposed approach that offers particular benefit when current practice is poor.Further research is essential to define the elements that should be included in bundles to improve the quality of care for CDI.
Using CDI as a Care Quality Indicator
The implementation and effectiveness of a CDI care bundle should ideally be measured within quality improvement frameworks using both process indicators (e.g. the percentage of patients who commence treatment on the same day as diagnosis and the percentage of patients treated according to current guidelines) and outcome indicators (in-hospital or 30-day mortality and frequency of complications)
CDI is a healthcare facility-wide problem linked to all factors responsible for HAIs in general, including antimicrobial use an overuse, improper or inadequate handwashing, lapses in infection control procedures, poor decontamination of the healthcare environment, a lack of education and training and understaffing. The incidence of healthcare-associated CDI itself is a potentially useful routine marker of care quality and patient safety and yet it is little used in Europe. It has only been used as a national performance measure, and is mandatorily and publicly reported at hospital level, in the UK (since 2004) (Public Health England 2015; Department of Health 2012). In Ireland, CDI has been an indicator in the national service plan since 2013 (F. Fitzpatrick, personal communication). Public reporting of CDI is also mandatory in Canada where in common with the UK it has been accompanied by a reduction in cases (Daneman et al. 2012). We would argue that mandatory, centralised, national collection and public reporting of hospital-level CDI incidence rates (with the origin or case, i.e. healthcare-associated or community-associated) would benefit CDI control, while acknowledging that such data should be collected using standardized methods and subject to risk adjustment and external audit.
There is a consensus among European infectious disease experts that public reporting of HAI indicators can benefit hospitals (Martin et al. 2013). Whether they directly benefit or influence patients themselves, and how indicators should be chosen and implemented, are more contentious questions. In 2009 the European Council recommended the development of a set of reliable and comparable patient safety indicators to help identify safety problems, evaluate the effectiveness of interventions aimed at improving safety, and facilitate mutual learning between Member States (Council of the European Union 2009). National performance indicators for HAIs in hospitals have since been developed by collaborative European projects (Cookson et al. 2011). However, according to the European Commission, in 2012 data on a limited and variable set of HAI indicators were made publicly available at the hospital level only in Denmark, France, Ireland, Luxembourg, Norway and the UK (European Commission 2012).
Generally, infection control experts are reported to favour structure indicators (e.g. the existence of national programmes, committees, guidelines and resources) and process indicators (e.g. alcohol-based hand-rub consumption) rather than outcome indicators (e.g. HAI incidence rates) (Martin et al. 2013). Concerns regarding outcomes data include the potential for mis-interpretation (due for example to intersite differences in the patients monitored or the surveillance methods used) and under-reporting. Experts in France have proposed conditions for implementing and reporting performance indicators, namely a broad debate on their benefits and drawbacks, a test period, the avoidance of extra workload for infection control teams and the use of quality and validity control measures (French Society for Hospital Hygiene 2013).
Conclusion
There is now clear evidence that CDI increases the risk of death in hospitalised patients, especially among vulnerable groups such as the elderly and immunocompromised. This substantial and often preventable threat to patient safety warrants specific attention by healthcare policymakers, and this will become important as the population ages. Addressing CDI is not a matter for microbiologists and infection specialists alone, as the infection threatens patients in all medical specialties and sites of care. It requires organizational leadership and commitment, with systemwide prioritisation of prompt and accurate diagnosis, and the implementation and audit of guidelines-led treatment and infection control measures, and continued European, national and local surveillance. Care bundles designed to improve CDI management and avoid complications, recurrence and mortality should be implemented within frameworks for improving quality and patient safety and monitored using appropriate indicators. We would also argue that mandatory centralized national collection and public reporting of hospital-level CDI incidence rates would benefit CDI control.
Conflict of Interest
FB has received fees as speaker and member of advisory boards from Pfizer, Novartis, Astellas Pharma Europe Ltd, MSD, Cepheid, bioMérieux and Summit. JC has received fees as speaker and member of advisory boards from Astellas Pharma Europe Ltd, AstraZeneca, Pfizer and Novartis. OAC is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN1106) and the European Commission, and has received research grants from, is an advisor to, or received lecture honoraria from Actelion, Astellas Pharma Europe Ltd Cubist, Genzyme, MSD, Optimer, Sanofi Pasteur, Summit, and Viropharma. EJK has received fees as speaker and/or member of advisory boards from Pfizer, Novartis, Astellas Pharma Europe Ltd and MSD. NP has received fees as speaker and member of advisory boards from Pfizer, Novartis, Astellas Pharma Europe Ltd, MSD, Johnson & Johnson and Carefusion.
Authors’ Contributions
This paper was born from discussions at meetings of CDI Europe, an expert-led initiative aiming to translate research on CDI into meaningful policy responses to help improve patient outcomes, supported by Medline literature searches using relevant key words. All authors were involved at each stage, from the initial proposal, through review and input into the outline and multiple drafts to final approval.
Acknowledgements
All authors are members of the CDI Europe group. This manuscript was reviewed by the following additional members of the CDI Europe group: Mark Wilcox (Leeds, UK: Chair), Franz Allerberger (Vienna, Austria), Fidelma Fitzpatrick (Dublin, Ireland), Elisabeth Nagy (Szeged, Hungary), Carl Eric Nord (Stockholm, Sweden), Maja Rupnik (Maribor, Slovenia).
Editorial assistance was provided by Interel Ltd., funded by Astellas Pharma Europe Ltd. Astellas Pharma Europe Ltd provided editorial assistance for the final paper by way of a factual accuracy check only.
The development of this paper was supported by Astellas Pharma Europe Ltd. Medical Writer attendance at the review meetings was funded by Astellas Pharma Europe Ltd, as was all subsequent time spent working with the authors to produce and submit the manuscript. The views expressed are those of the authors and not necessarily of Astellas Pharma Europe Ltd.
Abbreviations
CDI Clostridium difficile infection
CDC Centers for Disease Control and Prevention
CI confidence intervals
ECDC European Centre for Disease Prevention and Control
HAI healthcare-associated infection
References:
Abbett SK, Yokoe DS, Lipsitz SR et al. (2009) Proposed checklist of hospital interventions to decrease the incidence of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol, 30(11): 1062-9.PubMed ↗
Asensio A, Di Bella S, Lo Vecchio A et al. (2015) The impact of Clostridium difficile infection on resource use and costs in hospitals in Spain and Italy: a matched cohort study. Int J Infect Dis, 36: 31-8. PubMed ↗
Barbut F, Surgers L, Eckert C et al. (2014) Does a rapid diagnosis of Clostridium difficile infection impact on quality of patient management? Clin Microbiol Infect, 20(2): 136-44. PubMed ↗
Bauer MP, Notermans DW, van Benthem BH et al. (2011) Clostridium difficile infection in Europe: a hospital-based survey. Lancet, 377: 63-73. PubMed ↗
Berman L, Carling T, Fitzgerald TN et al. (2008) Defining surgical therapy for pseudomembranous colitis with toxic megacolon. J Clin Gastroenterol, 42(5): 476-80. PubMed ↗
Bhangu A, Nepogodiev D, Gupta A et al. (2012) Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg, 99(11): 1501-13. PubMed ↗
Centres for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States. Atlanta: CDC. [Accessed: 18 April 2016] Available from http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
Committee on the Environment, Public Health and Food Safety (Rapporteur: Oreste Rossi) (2013) Report on the report from the Commission to the Council on the basis of Member States' reports on the implementation of the Council Recommendation (2009/C 151/01) on patient safety, including the prevention and control of healthcare-associated infections [Procedure file 2013/2022(INI)]. Brussels: European Parliament. [Accessed: 18 April 2016] Available from http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+REPORT+A7-2013-0320+0+DOC+XML+V0//EN
Cookson B, Mackenzie D, Coutinho AP et al. (2011) Consensus standards and performance indicators for prevention and control of healthcare-associated infection in Europe. J Hosp Infect, 79(3): 260-4. PubMed ↗
Council of the European Union (2009) Council recommendation of 9 June 2009 on patient safety, including the prevention and control of healthcare associated infections. Official Journal of the European Union, 52: Notice 2009/C 151/01.
Council of the European Union (2014) Council conclusions on patient safety and quality of care, including the prevention and control of healthcare associated infections and antimicrobial resistance. Brussels: Press office - General Secretariat of the Council. [Accessed: 18 April 2016] Available from http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/lsa/145976.pdf.
Crobach MJL, Dekkers OM, Wilcox MH et al. (2009) European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect, 15(12): 1053-66. PubMed ↗
Curtin BF, Zarbalian Y, Flasar MH et al (2013) Clostridium difficile-associated disease: adherence with current guidelines at a tertiary medical center. World J Gastroenterol, 19(46): 8647-51. PubMed ↗
Daneman N, Stukel TA, Ma X et al. (2012) Reduction in Clostridium difficile infection rates after mandatory hospital public reporting: findings from a longitudinal cohort study in Canada. PLoS Med, 9(7): e1001268. PubMed ↗
Davies KA, Longshaw CM, Davis GL et al. (2014) Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis, 14(12): 1208-19. PubMedc↗
Debast SB, Bauer MP, Kuijper EJ et al. (2014) European Society of Clinical Microbiology and Infectious Diseases (ESCMID): update of the treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect, 20 Suppl 2: 1-26. PubMed ↗
Department of Health (2012) The NHS outcomes framework 2013-14. London: Department of Health. [Accessed: 18 April 2016] Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213055/121109-NHS-Outcomes-Framework-2013-14.pdf
Department of Health (2010) High impact intervention care bundle to reduce the risk from Clostridium difficile. London: Department of Health [Accessed: 18 April 2016] Available from http://webarchive.nationalarchives.gov.uk/20120118164404/http://hcai.dh.gov.uk/files/2011/03/Document_Clostridium_difficile_Infection_High_Impact_Intervention_FINAL_101210.pdf
Dubberke ER, Carling P, Carrico Ret al. (2014) Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol, 35: 628-45. PubMed ↗
European Centre for Disease Prevention and Control (2013) Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC. [Accessed: 18 April 2016] Available from http://ecdc.europa.eu/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf
European Commission (2012) Commission staff working document: detailed analysis of countries’ reports on the implementation of the Council Recommendation (2009/C 151/01) on patient safety, including the prevention and control of healthcare associated infections. SWD(2012) 366 final. Brussels: European Commission. [Accessed: 18 April 2016] Available from http://ec.europa.eu/health/patient_safety/docs/council_2009_report_en.pdf
European Commission (2014) Report from the commission to the Council. The Commission’s second report to the Council on the implementation of Council recommendation 2009/C 151/01 on patient safety, including the prevention and control of healthcare associated infections (COM(2014) 371). Brussels: European Commission. [Accessed: 18 April 2016] Available from http://ec.europa.eu/transparency/regdoc/rep/1/2014/EN/1-2014-371-EN-F1-1.Pdf
Feazel LM, Malhotra A, Perencevich EN et al. (2014) Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother, 69(7): 1748-54. PubMed ↗
French Society for Hospital Hygiene (SF2H), Lucet JC, Parneix P et al. (2013) Should public reporting be made for performance indicators on healthcare-associated infections? Med Mal Infect, 43(3): 108-13. PubMed ↗
Garner MJ, Carson C, Lingohr EJ et al. (2015) An assessment of antimicrobial resistant disease threats in Canada. PLoS One, 10: e0125155. PubMed ↗
Hall AJ, Curns AT, McDonald LC et al. (2012) The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis, 55(2): 216-23. PubMed ↗
Heimann SM, Vehreschild JJ, Cornely OA et al. (2015) Economic burden of Clostridium difficile associated diarrhoea: a cost-of-illness study from a German tertiary care hospital. Infection, 43(6): 707-14.PubMed ↗
Hensgens MP, Goorhuis A, Dekkers OM et al. (2013) All-cause and disease-specific mortality in hospitalized patients with Clostridium difficile infection: a multicenter cohort study. Clin Infect Dis, 56: 1108-16. PubMed↗
Jardin CG, Palmer HR, Shah DN et al. (2013) Assessment of treatment patterns and patient outcomes before vs after implementation of a severity-based Clostridium difficile infection treatment policy. J Hosp Infect, 85(1): 28-32. PubMed ↗
Jury LA, Tomas M, Kundrapu S et al. (2013) A Clostridium difficile infection (CDI) stewardship initiative improves adherence to practice guidelines for management of CDI. Infect Control Hosp Epidemiol, 34(1): 1222-4. PubMed ↗
Kim ES, Kim YJ, Park CW et al. (2013) Response failure to the treatment of Clostridium difficile infection and its impact on 30-day mortality. Hepatogastroenterology, 60(123): 543-8. PubMed ↗
Kwok CS, Arthur AK, Anibueze CI et al. (2012) Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol, 107(7): 1011-9.
<http://www.ncbi.nlm.nih.gov/pubmed/22525304> PubMed
Le F, Arora V, Shah DN et al. (2012) A real-world evaluation of oral vancomycin for severe Clostridium difficile infection: implications for antibiotic stewardship programs. Pharmacotherapy, 32(2): 129-34. PubMed ↗
Le Monnier A, Duburcq A, Zahar JR et al. (2015) Hospital cost of Clostridium difficile infection including the contribution of recurrences in French acute-care hospitals. J Hosp Infect, 91(2): 117-22. PubMed ↗
Lessa FC, Mu Y, Bamberg WM et al. (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med, 372(9): 825-34. PubMed ↗
Lim SK, Stuart RL, Mackin KE et al. (2014) Emergence of a ribotype 244 strain of Clostridium difficile associated with severe disease and related to the epidemic ribotype 027 strain. Clin Infect Dis, 58(12): 1723-3.PubMed ↗
Magill SS, Edwards JR, Bamberg W et al. (2014) Multistate point-prevalence survey of health care-associated infections. N Engl J Med, 370(13): 1198-208. PubMed ↗
Martin M, Zingg W, Hansen S et al. (2013) Public reporting of healthcare-associated infection data in Europe. What are the views of infection prevention opinion leaders? J Hosp Infect, 83(2): 94-8. PubMed ↗
Muto CA, Blank MK, Marsh JW et al. (2007) Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive "bundle" approach. Clin Infect Dis, 45(10): 1266-73. PubMed ↗
Office for National Statistics (2013) Deaths involving Clostridium difficile - Reference tables, 1999 and 2001 to 2012. Number of deaths and age-standardised mortality rates for deaths where Clostridium difficile was recorded as the underlying cause or mentioned anywhere on the death certificate, in England and Wales, for 1999 and 2001 to 2012. London: ONS. [Accessed: 18 April 2016] Available from http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/rel/subnational-health2/deaths-involving-clostridium-difficile/2012/reference-tables.xls.
Oldfield EC, Oldfield EC, Johnson DA. (2014) Clinical update for the diagnosis and treatment of Clostridium difficile infection. World J Gastrointest Pharmacol Ther, 6: 1-26.
Planche TD, Davies KA, Coen PG et al. (2013) Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis, 13: 936-45. PubMed ↗
Public Health England (2015) Financial year counts and rates of C. difficile infection by NHS acute trust (FY 2007/08 – FY 2013/14, Table 8a). [Accessed: 18 April 2016] Available from gov.uk/government/uploads/system/uploads/attachment_data/file/336953/Clostridium_difficile_annual_and_quarterly_trusts_and_ccgs.xls
Resar R, Griffin FA, Haraden C et al. (2012) Using care bundles to improve health care quality. IHI Innovation Series white paper. Cambridge, Massachusetts: Institute for Healthcare Improvement. [Accessed: 18 April 2016] Available from ihi.org/resources/Pages/IHIWhitePapers/UsingCareBundles.aspx.
Schmid D, Kuo HW, Simons E et al. (2013) All-cause mortality in hospitalized patients with infectious diarrhea: Clostridium difficile versus other enteric pathogens in Austria from 2008 to 2010. J Infect Public Health, 7(2): 133-44. PubMed ↗
Taori SK, Wroe A, Poxton IR (2013) Clostridium difficile infections in SE Scotland: mortality and recurrence in a region without PCR ribotype 027. J Med Microbiol, 62(Pt 9): 1468-77. PubMed ↗
UK National Audit Office (2009) Reducing healthcare associated infections in hospitals in England. London: National Audit Office. [Accessed: 18 April 2016] Available from https://www.nao.org.uk/report/reducing-healthcare-associated-infections-in-hospitals-in-england/
Vonberg RP, Kuijper EJ, Wilcox MH et al. (2008) Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect, 14 Suppl 5: 2-20. PubMed ↗
Walker AS, Eyre DW, Wyllie DH et al. (2013) Relationship between bacterial strain type, host biomarkers, and mortality in Clostridium difficile infection. Clin Infect Dis, 56(11): 1589-600. PubMed ↗
Wenisch JM, Schmid D, Tucek G et al. (2012) A prospective cohort study on hospital mortality due to Clostridium difficile infection. Infection, 40(5): 479-84. PubMed ↗
Wiegand PN, Nathwani D, Wilcox MH et al. (2012) Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect, 81(1): 1-14. PubMed ↗











