ICU Management & Practice, Volume 18 - Issue 4, 2018
In this article we focus on the evidence of whole-body ultrasonography used
for hypotension or shock. We first highlight individual ultrasound components
in association to hypotension and shock. Second, we provide an outline
of current literature on whole-body ultrasonography, its effect on outcome,
and try to integrate the previous observations.
Critical care ultrasonography (CCUS) is increasingly advocated and used, and is defined as point-of-care image acquisition, interpretation and clinical application, all performed by the critical care clinician, and directed to inform on specific clinical questions (Narasimhan et al. 2016). Recently, Zaidi and Koenig (2018) splendidly described the use of ultrasonography in critical care in this journal, where they advocate using methods of CCUS appropriate for each patient-specific problem. One of the frequent problems in critically ill patients is how to guide diagnostics and treatment in patients with hypotension or shock. In these patients diagnostic challenges focus on unravelling the underlying cause(s), and treatment challenges focus on the need and titration of fluids, vasopressors and inotropes. Hypotension can result from various underlying causes, such as cardiac failure, cardiac tamponade, tension pneumothorax, sepsis due to pneumonia, liver and spleen rupture due to blunt trauma and ruptured aortic aneurysm. To capture the entire scope of the problem or provide necessary clues for unravelling the underlying causes, whole-body ultrasonography has been advocated to be more valuable than single organ CCUS (Narasimhan et al 2016; Lichtenstein and Axler 1993).
Individual component ultrasonography
The individual components of whole-body ultrasonography have been extensively described (Frankel et al. 2015). Volpicelli et al. presented in their study on undifferentiated hypotension in the emergency department a protocol that summarises the views of the heart, lungs, inferior vena cava, (minimal) abdomen and peripheral veins with a focus on diagnosis (Volpicelli et al. 2013). Mok advocated the SIMPLE approach to manage patients with shock (Mok 2016). Balmert et al. made a systematic overview on ultrasonography in the acute setting (Balmert et al. 2018). They highlighted all the potential indications for ultrasonography and described recent innovations that enable assessment of organs in more detail, for example by strain imaging of the heart or measuring perfusion of the kidneys and liver. Our description of each organ focuses on the potential of ultrasonography to unravel underlying causes in patients with hypotension or shock as well as the potential to guide treatment.
Cardiac ultrasonography
The heart is one of the most important ultrasonography sites for identifying potential causes of hypotension. Different types of shock can be discriminated based on the information acquired from CCUS. The most striking example is a pericardial effusion in the presence of obstructive shock. Ultrasonography of the heart or echocardiography is often summarised, and numerous protocols have been developed (RACE [rapid assessment by cardiac echo]/RUSH [rapid ultrasound in shock]/FATE [focused assessed transthoracic echocardiography]) (Volpicelli et al. 2013; Perera et al. 2010; McLean and Huang 2012; Jensen et al. 2004; McLean 2016). The ultrasound signs are evaluated using basic level two-dimensional (2D) and M-mode ultrasonography. Underlying major cardiac pathology can be visualised using the orthodox parasternal, apical 4 - and 5 chamber and subcostal 4 chamber views identifying pericardial effusions, severely depressed contractility of the left (LV) and/or right ventricle (RV), (severe) dilatation of the LV and/or RV, or clues to intravascular volume status (e.g. “kissing ventricle”) (McLean and Huang 2012). Assessment of LV contractility can be done by measuring fractional shortening using M-mode (FS) and LV ejection fraction (LVEF) using eyeballing or the modified Simpson biplane method; eyeballing is the preferred method, but it requires training. Studies have shown that non-cardiologists could achieve good agreement on the visual estimation of LV ejection fraction with cardiologists (Unlüer et al. 2014; Randazzo et al. 2003; Bustam et al. 2014). Assessment of right ventricular function is challenging; the measurement of the Tricuspid Annular Plane Systolic Excursion (TAPSE) is often used to get a global estimation of right ventricular function, together with an impression of the presence or absence of right ventricular dilatation. More advanced techniques such as colour Doppler, tissue Doppler imaging and speckle tracking allow identification of (major) valve defects, LV diastolic dysfunction and more subtle analyses of cardiac dysfunction (i.e. Strain, RV S’). Measuring strain is a promising tool for early diagnosis of cardiac injuries that are non-detectable with conventional measurements (Boissier et al. 2017; Sanfilippo et al. 2017; Clancy et al. 2017; De Geer et al. 2015). Despite suboptimal positioning and hampered image quality in the critically ill, several studies have shown that these advanced techniques/measurements are viable in the critical care setting (Boissier et al. 2017; Sanfilippo et al. 2017; Clancy et al. 2017; De Geer et al. 2015).
The presence or absence of cardiac dysfunction may inform the clinician about the type of ([non-]cardiogenic) origin of the shock. However, in critically ill patients, diagnostic clues are not so straightforward and frequently patients present with a combination of types of shock. The cardiac ultrasonography findings ultimately have to be integrated into the overall assessment of the patient.
Besides diagnostic information, CCUS of the heart can also give more information on the haemodynamic profile at hand and guide treatment. For instance, an advanced measurement such as ultrasound-derived cardiac output can evaluate fluid responsiveness or the effects of inotropes. Kanji et al. (2014) suggested that echocardiography in patients with undifferentiated shock in the ICU guided towards treatment with less fluids and more dobutamine, which was associated with improved 28-day mortality and improved renal function. However, these results were obtained by a retrospective cohort without echocardiography and a prospective cohort with echocardiography.
Lung ultrasonography
Lung ultrasonography relies mainly on the detection of ultrasonography artefacts (Lichtenstein 2015). In the intercostal space the pleural line is seen as the hyperechoic line that moves upward and downward with ventilation, which is called lung sliding: the movement of the visceral with the parietal pleura (Goffi et al. 2018). The A-lines are hyperechoic horizontal lines and they are reverberation artefacts generated from the strong reflectivity of the pleural line. Along with lung sliding, they make up the normal view of lung tissue in ultrasonography (Miller 2016; Lichtenstein 2014). When the lung tissue increases in density, due to increased lung weight (e.g. accumulation of blood, lipids, pus or proteins, increased extravascular lung water, deposition of collagen and fibrotic tissue) or lung de-aeration (i.e. atelectasis), this is associated with the appearance of B-lines. B-lines are discrete laser-like vertical hyperechoic reverberation artefacts that arise from the pleural line and extend to the bottom of the screen without fading, erasing the A-lines (Volpicelli et al. 2012). It is considered a positive finding when there are three or more B-lines in a longitudinal plane between two ribs, because even two lines may be present in the normal lung. The presence of multiple B-lines is the sonographic sign of lung interstitial syndrome, which can be caused by pulmonary oedema, interstitial pneumonia and diffuse parenchymal lung disease (pulmonary fibrosis) (Covic et al. 2018). Furthermore, a focal (localised) sonographic pattern of interstitial syndrome may be seen in the presence of atelectasis, pulmonary contusion, pulmonary infarction, pleural disease and neoplasia.
Ultrasonography of the inferior vena cava
Normally the inferior vena cava (IVC) has a diameter with an average of around 20 mm and it will collapse slightly at inspiration and dilate again at expiration. Ferrada et al. (2012) found in 108 acutely admitted critically ill patients that guiding fluid treatment based on a IVC of 20 mm or smaller was associated with a decrease in lactate levels. By measuring the minimal and maximal diameter of the IVC, the Inferior Vena Cava–Collapsibility Index (IVC–CI) can be calculated (Brennan et al. 2007). A small IVC–CI would imply venous congestion, for there is too much intravascular fluid and the IVC can no longer collapse normally. A normal collapsibility is arbitrarily set at 40 to 50%, although the diagnostic accuracy is not perfect (Brennan et al. 2007; Lang et al. 2015). In mechanically ventilated patients the IVC–CI may not be as reliable, since intrathoracic pressure is mechanically increased (Ilyas et al. 2017). Citilcioglu et al. (2014) investigated the association between the IVC measured by bedside ultrasonography and CVP measured by a central venous catheter. In 45 patients the IVC diameter at both expiration and inspiration was associated with CVP in spontaneous breathing patients. In mechanically ventilated patients this association was not seen. Stawicki et al. (2009) showed similar results.
Liver and spleen ultrasonography
Liver Doppler ultrasonography may show signs of abnormalities in haemodynamic function (McNaughton et al. 2011). The splenic arterial and venous flow indices have similarly mostly been investigated in patients with liver disease (Baik 2010; Piscaglia et al. 2002), and no association with systemic haemodynamic function was found. One study by Bolognesi et al. (2012) evaluated the use of splenic ultrasonography in heart failure patients and found that splenic pulsatility index was associated with right arterial pressure and right ventricle end-diastolic pressure, suggesting that this measurement reflects congestion of the spleen. Characteristics of arterial and venous flow in the liver and spleen have been used mostly in specific disease, but could add to our understanding of venous congestion or shock in a more general population of critically ill patients.
Renal and bladder ultrasonography
Renal ultrasonography includes focused renal ultrasonography for evaluation of renal, pre- or post-renal pathology. The Renal Resistive Index (RRI) and Venous Impedance Index (VII) have been used in a variety of clinical settings. Doppler imaging identifies changes in blood flow at the microvascular level (Kelahan et al. 2018). Evaluation of changes in blood flow at different sites of the renal parenchyma could provide useful diagnostic and prognostic information for critically ill patients. An increase in RRI may be an early sensitive sign of haemodynamic deterioration, even in stable patients. Ninet et al. (2015) showed in a meta-analysis that an elevated RRI may be a predictor of persistent acute kidney injury in critically ill patients. The VII has only been evaluated in specific patient populations such as diabetic and heart failure patients (Jeong et al. 2011; Nijst et al. 2017). Potential limitations of these methods are whether RRI and VII can be obtained in such a manner that measures can be reproduced and whether it is feasible to measure VII in unstable, critically ill patients. To establish a more definite role for Doppler imaging of the kidney it should first be investigated in a large unselected group of critically ill patients. When imaging the bladder free fluid in the pelvis could be detected.
Ultrasonography of the aorta
The abdominal aorta can be investigated along the midline of the abdomen. The normal size of the abdominal aorta should be less than 30 mm. A larger abdominal aorta is suggestive of an aneurysm. Rupture of the abdominal aorta might be identified as the cause of hypotension or shock. The diagnostic accuracy of CCUS is very high for acute aneurysm.
Whole-body ultrasonography
The benefit of whole-body ultrasonography has been highlighted by cases presented in the literature (Schmidt et al. 2016; Mosier 2014). In these cases, a stepwise approach is most often used to unravel the underlying cause of hypotension or shock. The simplest benefit of whole-body ultrasonography over single organ ultrasonography results for instance from measuring the cardiac output and investigating the presence or absence of alveolar oedema in one patient, which may narrow down the differential diagnosis of shock significantly (Cecconi et al. 2014). A stepwise approach of many organs in one examination may result in a standard protocol such as RACE for echocardiography, as noted previously. Many protocols have been created to evaluate multiple organs using ultrasonography, including ACES (abdominal and cardiac evaluation with sonography in shock), FATE (focused assessed transthoracic echocardiography), SIMPLE, RUSH (rapid ultrasound in shock) and SEARCH 8Es (Sonographic Evaluation of Aetiology for Respiratory difficulty, Chest pain, and/or Hypotension) (Mok 2016; Perera et al. 2010; Ahn et al. 2017; Atkinson et al. 2008; Skrzypek et al. 2018). Each of these protocols contains more or less the same core ultrasound components. The SIMPLE protocol additionally focuses on intramural mass and intimate flap. The most important difference between these protocols is the order of procedure priority and the specific focus.
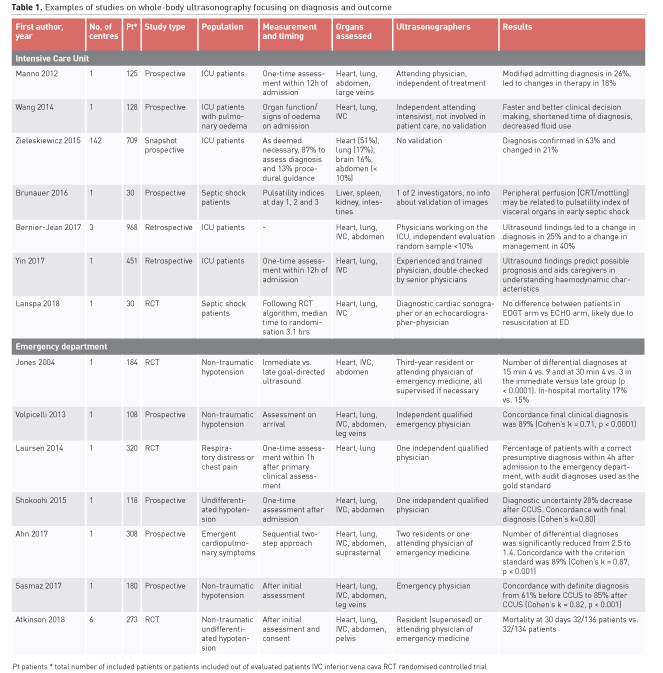
The first study published on whole-body ultrasound in the ICU came from Lichtenstein and Axler (1993). In 150 consecutive patients they visualised the abdomen, pleural space, peritoneal cavities, great vessels, bile ducts, urinary and gastrointestinal tract and femoral veins. In 33 (22%) patients ultrasonic positive findings contributed to the immediate management. Thereafter, an increasing number of studies, with number of patients ranging from case series to a cohort of up to 1000 patients, focused on the use of multi-organ or whole-body ultrasonography for evaluating hypotension or shock (Table 1). Irrespective of the setting whole-body ultrasonography seems to increase the number of patients with a definite diagnosis or to have implications on treatment as compared to patients in whom whole-body ultrasonography is not performed. Other measures were a decrease in diagnostic uncertainty and time to diagnosis. In the emergency department two studies even randomised patients to different strategies (Jones et al. 2004; Laursen et al. 2014). Early ultrasonography seems to result in a right diagnosis at an earlier time. The Sonography in Hypotension and Cardiac Arrest in the Emergency Department (SHoC-ED) trial investigated whether randomisation to whole-body ultrasonography compared to standard work-up without ultrasonography was associated with a better outcome in the emergency room and found equal survival after 30 days (Atkinson et al. 2018). Interestingly, despite the benefit for establishing a diagnosis in a larger number of patients treatment was equal (Atkinson et al. 2018).
Challenges
The primary challenge concerns technical difficulties of the measurements and the highly operator-dependent method of obtaining the measurements. In the case of whole-body ultrasonography various individual components should be obtained and interpreted. For the purpose of a concise but complete assessment, measurements need to be simple to perform and interpret. Furthermore, the understanding and integration of ultrasonography findings in relation to other patient-derived parameters needs further investigation (Frankel et al. 2015). Another challenge concerns the interpretation of large amounts of data that are generated when repeatedly performing whole-body ultrasonography. There are developments in the areas of machine learning and artificial intelligence that could aid in interpretation and/or analysis of data generated in these exams, but these are not yet fully developed to be used in daily practice. Last, as more and more technical possibilities arise in the ICU it is difficult to evaluate the precise additional value of CCUS in deliberating diagnosis. However, it is fast, noninvasive and supposedly simple, and deserves to be investigated.
Conclusion
We think that a mono-organ focus in unravelling disease states, such as ultrasonography of only the heart or the kidney, results in less clear understanding of the pathophysiologic state in critically ill patients as compared to a wide, open focus. Multiple protocols of integrated CCUS assessments exist, but improving techniques allow for more and more ultrasonographic possibilities. Interpretation of this integrated assessment may improve with increasing knowledge in this subject and become more universal. As new measurements and approaches continue to be investigated our diagnostic and prognostic accuracy will improve. We consider the whole-body and system focus important.
Conflict of interest
Renske Wiersema declares that she has no conflict of interest.
Geert Koster declares that he has no conflict of interest.
Iwan C.C. van der Horst declares that he has no conflict of interest.
Abbreviations
CCUS critical care
ultrasonography
IVC inferior vena cava
IVC–CI Inferior Vena Cava–Collapsibility Index
LV left ventricle
RRI Renal Resistive Index
RV right ventricle
VII Venous Impedance Index









