ICU Management & Practice, ICU Volume 14 - Issue 2 - Summer 2014
Authors
David Osman, MD
Medical Intensive Care Unit
Paris-South University Hospitals
Assistance Publique-Hôpitaux de Paris
Le Kremlin Bicêtre, France

Isabelle Boytchev, MD
Department of Gastroenterology
Paris-South University Hospitals
Assistance Publique-Hôpitaux de Paris
Le Kremlin Bicêtre, France
Intensivists are regularly confronted with the question of upper gastrointestinal bleeding, the mortality of which remains high, but should be reduced through recent diagnostic and therapeutic advances.
Introduction
Upper gastrointestinal bleeding (UGIB) is defined as a recent and sudden onset of haemorrhage originating from the oropharynx to the ligament of Treitz. In clinical practice, identification of lower versus UGIB can be difficult. UGIB usually presents as fresh blood or coffee ground haematemesis and/or melaena, but haematochezia may be the presenting sign in patients with massive bleeding. In a few cases, the haemorrhage is not overt and symptoms consist of a more or less severe anaemic syndrome. UGIB is usually divided into portal hypertension-related and unrelated causes. Peptic ulcer disease is the most common cause. UGIB is associated with a wide variety of severities, but remains a severe condition. Its mortality has probably not changed for 20 years, but could be reduced through recent diagnostic and therapeutic advances.
Management Before Endoscopic Diagnosis
- Delay Before Endoscopy
For overt or suspected UGIB, an oesophagogastroduodenoscopy (OGD) should always be performed (Barkun et al. 2010; Osman et al. 2013; de Franchis 2010). Its performance within 24 hours after admission was shown to be associated with a reduction in transfusion, second endoscopy and surgery requirement (Barkun et al.2010). When variceal bleeding is suspected, OGD is recommended in the first 12 hours (de Franchis 2010; Garcia-Tsao et al. 2010).
Earlier OGD (within 6 to 12 hours) is a matter of debate. A meta-analysis of three randomised trials showed no benefit of early endoscopy (Barkun et al. 2010). However, when active bleeding is suspected, early endoscopy may prove valuable and is usually recommended (Osman et al. 2013). One study identified fresh blood in gastric aspirates, haemodynamic instability and haemoglobin concentrate (Hb) <8 g/dL as predictors of the benefits of early OGD (Adamopoulos et al. 2003). A recent study demonstrated that in patients with a Glasgow-Blatchford score ≥ 12 the mortality rate was lower when OGD was performed within the first 13 hours (Lim et al. 2011). Pending endoscopy, appropriate management includes risk stratification (Srygley et al. 2012), pharmacological therapy and in some cases abdominal CT angiography.
- Risk Stratification
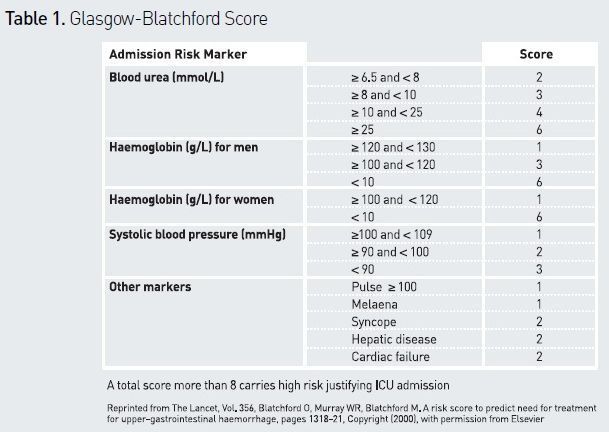
Several scoring systems have been described. The Rockall score was found to be a good indicator of the risk of rebleeding (Rockall et al. 1996), but it comprises only endoscopic data, and may therefore be of limited utility in the acute setting. The Glasgow-Blatchford score (see Table 1) includes clinical and biochemical data, and has proven useful in predicting the need for hospitalisation, transfusion, surgery or death (Blatchford et al. 2000). It is commonly used to identify patients at high risk and refer them to an intensive care unit (Barkun et al. 2010; Osman et al. 2013). An easily calculated risk score (AIMS65) has been developed to predict mortality, but needs further validation (Saltzman et al. 2011). The role of nasogastric tube and aspirate inspection for risk assessment is debated. Presence of red blood in aspirates suggests undeniably active bleeding and should prompt urgent OGD, but it should be remembered that absence of blood cannot rule out severe UGIB (Palamidessi et al. 2010).
- Pharmacological Therapy
Ulcer is the main cause of UGIB, and early administration of acid suppressive therapy is probably always reasonable (Osman et al. 2013). Pump proton inhibitors (PPIs) offer sustained and durable acid suppression. High dose of intravenous PPIs has become the dominant therapy for bleeding ulcers. A meta-analysis demonstrated that ‘standard’ doses of PPIs (in comparison with no treatment, placebo or H2-receptor antagonists) facilitated OGD by reducing the proportion of patients with active bleeding and the need for endoscopic haemostasis (Sreedharan et al. 2010). Another study established that administration of ‘high’ PPIs doses reduced transfusion requirement and rebleeding (Lau et al. 2007). This issue has not been examined by comparing ’high’ and ‘standard’ doses. Once portal hypertension is
suspected, a vasopressor agent active on the splanchnic circulation (terlipressin, somatostatin, somatostatin derivative) should be combined with PPis (Osman et al. 2013). By reducing portal hypertension, vasopressor agents stop variceal bleeding in 80% of cases (Garcia-Tsao et al. 2010), improve the quality of transport, and facilitate endoscopy (de Franchis 2010). To ensure emptying of gastric content, a prokinetic drug (erythromycin or metoclopramide) should be administered before OGD. A recent metaanalysis showed that the use of a prokinetic drug reduced the need for a second OGD (Barkun et al. 2010). If a nasogastric tube has been placed, gastric lavage is an effective alternative (Pateron et al. 2011).
- Role of CT Angiography
Management After Endoscopic Diagnosis of Ulcer Bleeding
Management of ulcer bleeding is well established, combining in most cases endoscopic and pharmacological therapies.
- Endoscopic Therapy
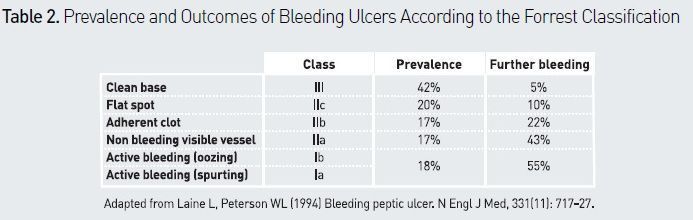
The Forrest classification (see Table 2) is used to categorise the appearance of bleeding ulcers and to determine the type of endoscopic treatment needed. The natural history of ulcer disease shows a rebleeding rate < 5% in the presence of a Forrest type IIc or III lesion (Gralnek et al. 2008). Endoscopic haemostasis should not be used in such cases (Barkun et al. 2010; Osman et al. 2013). Two meta-analyses confirmed the value of endoscopic therapy compared with PPIs alone in a high-risk population (Forrest Ia, Ib, IIa), by demonstrating a significant decrease in rebleeding (Barkun et al. 2009) and mortality (Leontiadis et al. 2006). It is now demonstrated that endoscopic treatment must include epinephrine injections and clips or thermal treatment, and not epinephrine alone (Laine et al. 2009). In Forrest type IIb lesions (adherent clot), endoscopic haemostasis is recommended when it seems possible, ie when the clot is small (Osman et al. 2013). A meta-analysis suggested that adherent clot should be removed in order to perform endoscopic treatment of the underlying artery lesion (Kahi et al. 2005).
- Pharmacological Therapy
In lesions at low risk of rebleeding, PPIs are recommended to be used at ‘standard’ doses. When a high risk is found (Ia to IIb), PPIs are usually recommended to be continued at ‘high’ doses for 72 hours (Barkun et al. 2010; Osman et al. 2013) even though in most studies ’high‘ doses were only compared to placebo (Lau et al. 2000; Leontiadis et al. 2007; Sung et al. 2009; Wang et al. 2010). The issue of Helicobacter pylori infection can rarely be resolved in the acute phase of UGIB, and there is probably no advantage in treating this infection on an emergency basis. If biopsy screening can be performed during the first OGD, without worsening bleeding, it is important to know that the sensitivity of rapid urease tests is lower in this setting (Tang et al. 2009). Although a meta-analysis clearly established that eradication of Helicobacter pylori reduced the long-term risk of recurrent bleeding, in comparison with antisecretory therapy alone (Gisbert et al. 2004), it has not been shown that eradication therapy was useful in case of early rebleeding.
Management After Endoscopic Diagnosis of Variceal Bleeding
Management of variceal bleeding includes in most cases endoscopic and pharmacological therapies.
- Endoscopic Therapy
Endoscopic haemostasis of bleeding oesophageal varices is based on band ligation rather than sclerosis (Gross et al. 2001). Obturation using cyanoacrylate glue is the reference treatment for bleeding gastric varices (Lo et al. 2001).
- Pharmacological Therapy
Combined treatment using vasoactive agents and endoscopic therapy has proven to be more effective than OGD alone in controlling bleeding (Sung et al. 1995; Avgerinos et al. 1997), and in survival without rebleeding (Besson et al. 1995; Cales et al. 2001). It is therefore recommended to continue vasoactive treatment using terlipressin or somatostatin or a somatostatin derivative for a period of three to five days after endoscopic therapy (de Franchis 2010; Osman et al. 2013). Early introduction of beta-blockers avoids rebound portal hypertension. A metaanalysis showed that combined treatment (ligation and beta-blocker introduced within a period of three days) significantly reduced rebleeding in comparison with endoscopic therapy or pharmacological therapy alone (Gonzalez et al. 2008).
Bacterial infections are observed in about 40%
of cirrhotic patients in the seven days following their admission for UGIB (Bernard
et al. 1995), and are independently associated with rebleeding and mortality (Goulis
et al. 1998). A meta-analysis established that antibiotic prophylaxis significantly
reduced mortality (Bernard et al. 1999). Third-generation cephalosporin or
fluoroquinolone for five to seven days is generally recommended to be given to
any cirrhotic patient with UGIB (de Franchis 2010; Osman et al. 2013).
Challenges and Perspectives
- Persistent and Recurrent bleeding
Treatment failure for UGIB covers two different aspects: persistent bleeding after haemostasis attempts and recurrence after primary success. From all causes, persistent bleeding occurs in approximately 10% of patients. The problem is particularly striking in Forrest Ia and Ib ulcer bleeding, where percutaneous arterial embolisation was demonstrated to be effective and is now recommended as a first-intention treatment (Osman et al. 2013). An analysis of 35 studies demonstrated that technical and clinical success rates of embolisation ranged from about 50% to 100% (Mirsadraee et al. 2011). Comparisons between surgery and embolisation showed equivalent results, although embolisation was applied to an older population. Interestingly, it has been shown that endoscopic marking with a metallic clip prior to embolisation enhanced the possibility of embolising the correct vessel (Eriksson et al. 2006). In variceal bleeding two studies demonstrated that early placement of a transjugular intrahepatic portosystemic shunt (TIPS) reduces the risk of persistent bleeding and rebleeding (Monescillo et al. 2004; Garcia Pagan et al. 2010). Improvement of survival following TIPS was also demonstrated in high-risk patients, defined as Child-Pugh class B patients with persistent bleeding at the time of OGD or Child-Pugh class C patients (Garcia Pagan et al. 2010). After endoscopic haemostasis of variceal bleeding, TIPS placement within 72 hours should therefore be considered in such patients (Osman et al. 2013).
In case of recurrent bleeding, whatever the cause, a second endoscopic attempt should first be proposed. Another approach is performing a second-look OGD in order to pre-empt recurrent bleeding. Second-look OGD is defined as an endoscopy scheduled for 16 to 24 hours after the initial OGD. A meta-analysis demonstrated in bleeding ulcer that second-look OGD with thermal coagulation reduced recurrent bleeding (without impact on the need for surgery, or mortality), but that second-look OGD with adrenaline injection had no beneficial effect (Tsoi et al. 2010). Another metaanalysis suggested that in ulcer bleeding, second-look endoscopy reduced the risk of rebleeding and surgery, but not mortality (Barkun et al. 2010). Therefore, recent guidelines proposed performing a secondlook OGD in ulcer bleeding when highrisk stigmata have been observed (Osman et al. 2013). Evaluation of the clinical impact of a strategy involving pre-emptive embolisation after initial endoscopic control of ulcer bleeding is ongoing.
- Management of Antithrombotic Therapy
Management of antiplatelet therapy in patients with UGIB is a clinical challenge. The decision to withhold or continue treatment should be discussed as soon as possible in a multidisciplinary setting. In ischaemic heart disease this issue is quite well codified. A meta-analysis demonstrated that discontinuing or not adhering to aspirin was associated with a three-fold higher risk of major cardiac events (Biondi-Zoccai et al. 2006). A randomised study in 156 patients with aspirininduced ulcer bleeding receiving endoscopic therapy and PPIs showed that immediate reintroduction of aspirin was associated with a non-significant increased risk of rebleeding, while discontinuation of aspirin was associated with a significant increase in eight weeks’ mortality (Sung et al. 2010). Consequently, in patients treated with antiplatelet therapy for ischaemic heart disease with UGIB, it is usually recommended to maintain aspirin (Osman et al. 2013). In dual antiplatelet therapy, clopidogrel is usually stopped until consultation with specialists.
The new generation of oral anticoagulants (nOAC) might be associated with higher UGIB risk, especially in patients with altered renal function. It is important to note that no established antidote is available in cases of nOAC that complicate serious bleeding. Prothrombin complex concentrates and recombinant factor VIIa may improve haemostasis in patients in whom bleeding develops during treatment with a nOAC, but their efficacy is unproven.
- Transfusion Management
The issue of transfusion strategy in UGIB is still poorly codified. Most guidelines recommend a policy of restricted blood transfusion (Barkun et al. 2010, de Franchis 2010, Osman et al. 2013). Transfusion management is particularly complex in cirrhotic patients, in whom increase in plasma volume seems to be linearly related to increase in portal pressure (Castaneda et al. 2000), encouraging particular prudence during the resuscitation of such patients. A recent trial demonstrated that UGIB patients randomised to receive transfusion to an Hb of 9 g/dl had a significantly higher rebleeding rate and mortality, in comparison with patients allocated to receive transfusion with the objective of 7g/dl (Villanueva et al). The difference in survival was mainly observed in cirrhotic Child–Pugh class A or B patients. Platelet transfusion in severe bleeding is usually recommended when platelet count is < 50,000/mm3 (Rossaint et al. 2010; Souweine et al. 2010). No study has examined this question in the particular setting of UGIB in cirrhotic patients, where the risk of worsening portal hypertension has also been raised (Colle et al. 2011). Moreover, thrombocytopaenia is common during cirrhosis and is a poor indicator of haemorrhagic risk (de Franchis 2010). On the basis of these arguments, in cirrhotic patients, platelet transfusion is usually recommended for a platelet count < 30,000/mm3 and should not delay endoscopy.
In haemorrhagic shock due to trauma early
treatment with fresh frozen plasma is recommended in massive bleeding (Rossaint
R, Crit Care 2010). Again, and for the same reasons, it is much debated in cirrhotic
patients (de Franchis 2010, Colle et al. 2011). Moreover, it is important to note
that neither prothrombin time (PT), nor international normalised ratio (INR) are
good indicators for coagulability in patients with cirrhosis. Administration of
fresh frozen plasma, with the objective of correcting a coagulopathy, is
therefore not recommended in cirrhotic patients with UGIB (Osman et al. 2013).
Conclusion
The management of patients with UGIB has significantly evolved throughout the past decade, and requires a multidisciplinary approach integrating pharmacological, endoscopic, and radiological options. Surgical treatment has become extremely rare. Future research is nevertheless needed to improve outcome in patients at high risk of rebleeding and to resolve current areas of uncertainty regarding transfusion and antithrombotic therapy management.
References:
Adamopoulos AB, Baibas NM, Efstathiou SP et al. (2003) Differentiation between patients with acute upper gastrointestinal bleeding who need early urgent upper gastrointestinal endoscopy and those who do not. A prospective study. Eur J Gastroenterol Hepatol, 15: 381-7.
Avgerinos A, Nevens F, Raptis S, Fevery J (1997) Early administration of somatostatin and efficacy of sclerotherapy in acute oesophageal variceal bleeds: the European Acute Bleeding Oesophageal Variceal Episodes (ABOVE) randomised trial. Lancet, 350: 1495-9.
Barkun AN, Bardou M, Kuipers EJ et al. (2010) International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med, 152: 101-13.
Barkun A, Wyse J, Romagnuolo J et al. (2009) Should we be performing routine second-look endoscopy in acute peptic ulcer bleeding in 2009? A meta-analysis. Gastroenterology, 134.
Bernard B, Cadranel JF, Valla D et al. (1995) Prognostic significance of bacterial infection in bleeding cirrhotic patients: a prospective study. Gastroenterology, 108: 1828-34.
Besson I, Ingrand P, Person B et al. (1995) Sclerotherapy with or without octreotide for acute variceal bleeding. N Engl J Med, 333: 555-60.
Biondi-Zoccai GG, Lotrionte M, Agostoni P et al. (2006) A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J, 27: 2667-74.
Blatchford O, Murray WR, Blatchford M (2000) A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet, 356: 1318-21.
Burks JA Jr., Faries PL, Gravereaux EC et al. (2001) Endovascular repair of bleeding aortoenteric fistulas: a 5-year experience. J Vasc Surg, 34: 1055-9.
Calès P, Masliah C, Bernard B et al. (2001) Early administration of vapreotide for variceal bleeding in patients with cirrhosis. N Engl J Med, 344: 23-8.
Castañeda B, Debernardi-Venon W, Bandi JC et al. (2000) The role of portal pressure in the severity of bleeding in portal hypertensive rats. Hepatology, 31: 581-6.
Colle I, Wilmer A, Le Moine O et al. (2011) Upper gastrointestinal tract bleeding management: Belgian guidelines for adults and children. Acta Gastroenterol Belg 74: 45-66.
Duchat F, Soyer P, Boudiaf M et al. (2010) Multi-detector row CT of patients with acute intestinal bleeding: a new perspective using multiplanar and MIP reformations from submillimeter isotropic voxels. Abdom Imaging, 35: 296-305.
Eriksson LG, Sundbom M, Gustavsson S et al. (2006) Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J Vasc Interv Radiol, 17: 959-64.
de Franchis R (2010) Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol, 53: 762-8.
Garcia-Tsao G, Bosch J (2010) Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med, 362: 823-32.
Gisbert JP, Khorrami S, Carballo F et al. (2004) H. pylori eradication therapy vs. antisecretory non-eradication therapy (with or without longterm maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database Syst Rev, (2): CD004062.
Gonzalez R, Zamora J, Gomez-Camarero J et al. (2008) Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med 149: 109-22.
Goulis J, Armonis A, Patch D et al. (1998) Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology, 27: 1207-12.
Gralnek IM, Barkun AN, Bardou M (2008) Management of acute bleeding from a peptic ulcer. N Engl J Med, 359: 928-37.
Gross M, Schiemann U, Mühlhöfer A et al. (2001) Meta-analysis: efficacy of therapeutic regimens in ongoing variceal bleeding. Endoscopy, 33: 737-46.
Kahi CJ, Jensen DM, Sung JJ et al. (2005) Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: a metaanalysis. Gastroenterology, 129: 855-62.
Laine L, McQuaid KR (2009) Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol, 7: 33-47.
Lau JY, Leung WK, Wu JC et al. (2007) Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med, 356: 1631-40.
Lau JY, Sung JJ, Lee KK et al. (2000) Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med, 343: 310-6.
Leontiadis GI, Sharma VK, Howden CW (2006) Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev, (1): CD002094.
Lim LG, Ho KY, Chan YH et al. (2011) Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy, 43: 300-6.
Lo GH, Lai KH, Cheng JS et al. (2001) A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology, 33: 1060-4.
Mirsadraee S, Tirukonda P, Nicholson A et al. (2011) Embolization for non-variceal upper gastrointestinal tract haemorrhage: a systematic review. Clin Radiol, 66: 500-9.
Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L et al. (2004) Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology, 40: 793-801.
Osman D, Djibré M, Da Silva D et al. (2012) Management by the intensivist of gastrointestinal bleeding in adults and children. Ann Intensive Care, 2: 46.
Palamidessi N, Sinert R, Falzon L et al. (2010) Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med, 17: 126-32.
Pateron D, Vicaut E, Debuc E et al. (2011) Erythromycin infusion or gastric lavage for upper gastrointestinal bleeding: a multicenter randomized controlled trial. Ann Emerg Med, 57: 582-9.
Rockall TA, Logan RF, Devlin HB et al. (1996) Risk assessment after acute upper gastrointestinal haemorrhage. Gut, 38: 316-21.
Rossaint R, Bouillon B, Cerny V et al. (2010) Management of bleeding following major trauma: an updated European guideline. Crit Care, 14: R52.
Saltzman JR, Tabak YP, Hyett BH et al. (2011) A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc, 74: 1215-24.
Van der Linden T, Souweine B, Dupic L et al. (2012) Management of thrombocytopenia in the ICU (pregnancy excluded). Ann Intensive Care, 2: 42.
Sreedharan A, Martin J, Leontiadis GI et al. (2010) Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev, (7): CD005415.
Srygley FD, Gerardo CJ, Tran T et al. (2012) Does this patient have a severe upper gastrointestinal bleed? JAMA, 10: 1072-9.
Sung JJ, Chung SC, Yung MY et al. (1995) Prospective randomised study of effect of octreotide on rebleeding from oesophageal varices after endoscopic ligation. Lancet, 346: 1666-9.
Sung JJ, Barkun A, Kuipers EJ et al. (2009) Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med, 150: 455-64.
Sung JJ, Lau JY, Ching JY et al. (2010) Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med 152: 1-9.
Tang JH, Liu NJ, Cheng HT et al. (2009) Endoscopic diagnosis of Helicobacter pylori infection by rapid urease test in bleeding peptic ulcers: a prospective case-control study. J Clin Gastroenterol, 43:133-9.
Tsoi KK, Chan HC, Chiu PW et al. (2010) Second-look endoscopy with thermal coagulation or injections for peptic ulcer bleeding: a metaanalysis. J Gastroenterol Hepatol, 25: 8-13.
Villanueva C, Colomo A, Bosch A et al. (2013) Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med, 368: 11-21.
Wang CH, Ma MH, Chou HC et al. (2010) High-dose vs non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med, 170: 751-8.



















