In two trials, including one involving frail elderly patients, evening administration of blood pressure (BP)--lowering medications showed no clinical benefits over morning administration, according to research presented at ESC Congress 2024.
BP typically follows a circadian rhythm, peaking after waking and reaching its lowest point during sleep. Major cardiovascular events, such as myocardial infarction and stroke, are more strongly associated with high night-time BP. Prior studies on whether lowering night-time BP preferentially could reduce cardiovascular risk have shown mixed results.
The BedMed trial was conducted in the general primary-care population, and the BedMed-Frail trial in nursing-home residents. Both studies found that while evening dosing was safe, it offered no additional advantages.
The open-label, pragmatic BedMed trial involved Canadian primary-care patients without a history of glaucoma who were prescribed at least one once-daily antihypertensive medication. Participants were randomly assigned to take all amenable antihypertensives either in the morning or at bedtime. The primary outcome was major adverse cardiovascular events (MACE), defined as all-cause death, hospitalisation/emergency department (ED) visits for stroke, myocardial infarction/acute coronary syndrome, or congestive heart failure. Secondary outcomes included all-cause unplanned hospitalisation/ED visits and visual, cognitive, and fracture-related events.
The BedMed-Frail trial had a similar design but involved residents of Canadian continuing care wards, who were assigned to either bedtime dosing or usual care (primarily morning use). Additional secondary outcomes included skin ulceration and deteriorated cognition.
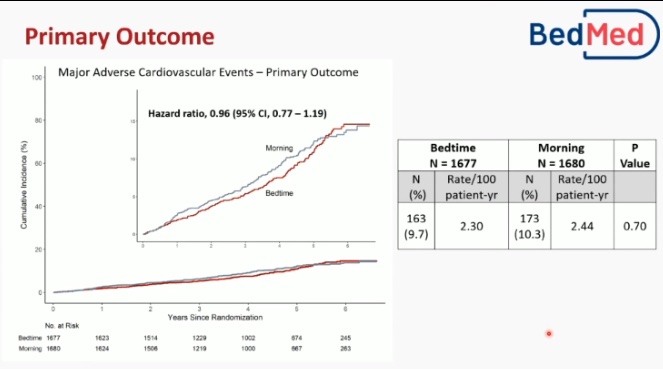
In the BedMed trial, 3,357 adults were randomised with a median age of 67 years, and 56% were female. Over a median follow-up of 4.6 years, the primary outcome of MACE occurred in 9.7% of participants in the bedtime group and 10.3% in the morning group. There were no differences in safety outcomes or all-cause hospitalisation/ED visits between the groups.
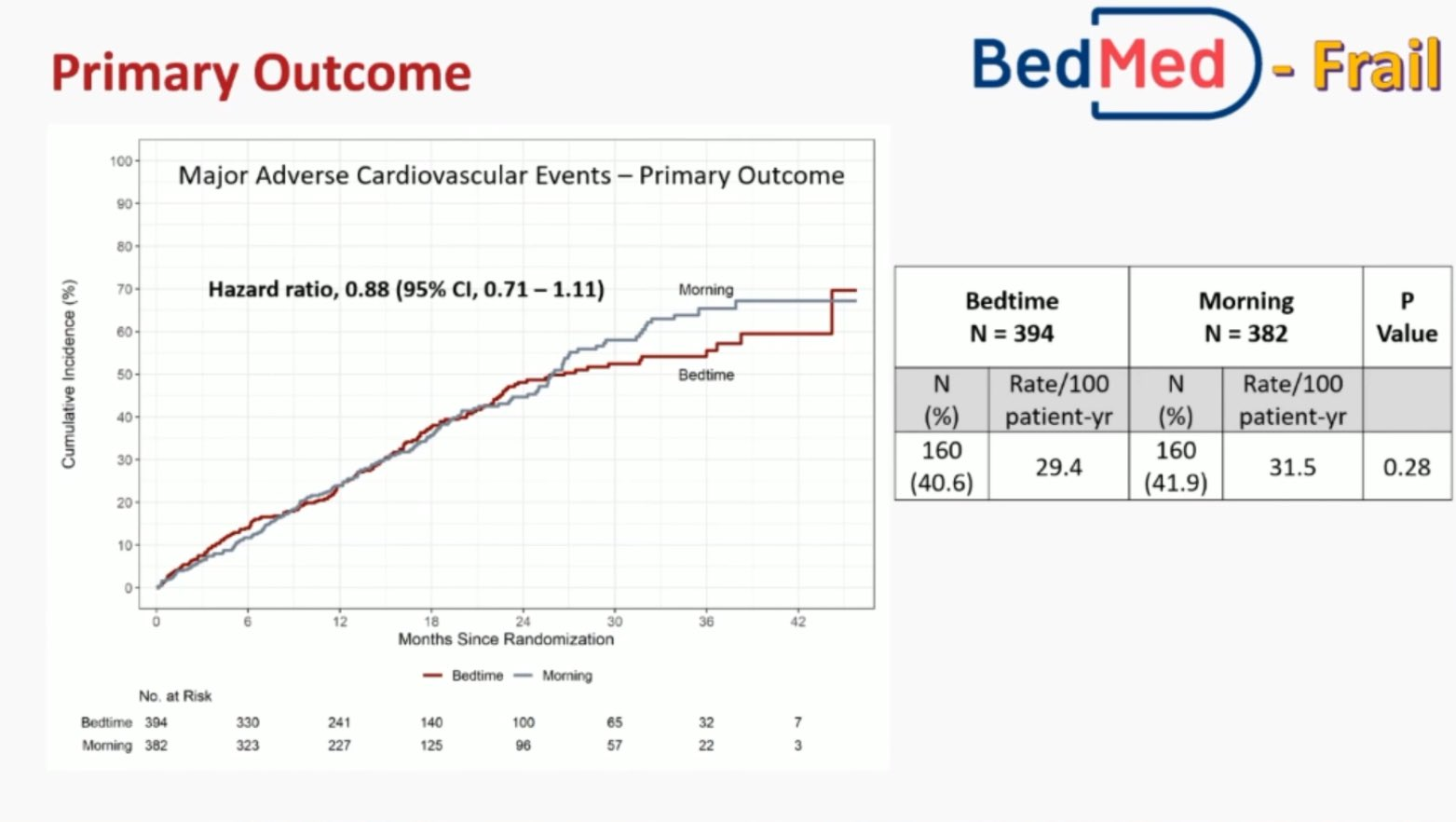
In the BedMed-Frail trial, 776 participants had a median age of 88 years, with 72% female. Over a median follow-up of 415 days, the primary outcome of MACE occurred in 40.6% of participants in the bedtime group and 41.9% in the usual care (morning) group, primarily driven by deaths in both groups. Secondary efficacy and safety outcomes were similar between groups, except for all-cause unplanned hospitalisation/ED visits, which favoured bedtime use.
Based on these findings, no difference was observed between bedtime and morning administration in terms of MACE, potential hypotensive, visual, cognitive, or other safety events in the general population and in frail older patients.
Source, Slides and Image Credit: ESC Congress 2024