Vutrisiran significantly reduced mortality, cardiovascular events, and markers of disease progression in patients with transthyretin amyloidosis with cardiomyopathy (ATTR-CM), according to late-breaking research presented today in a Hot Line session at ESC Congress 2024.
ATTR is a progressive, fatal disease characterised by the accumulation of misfolded transthyretin protein as amyloid deposits in various parts of the body, often affecting the heart. This study explored whether vutrisiran, a novel RNA interference (RNAi) therapeutic that targets transthyretin production, could improve clinical outcomes in patients with ATTR-CM.
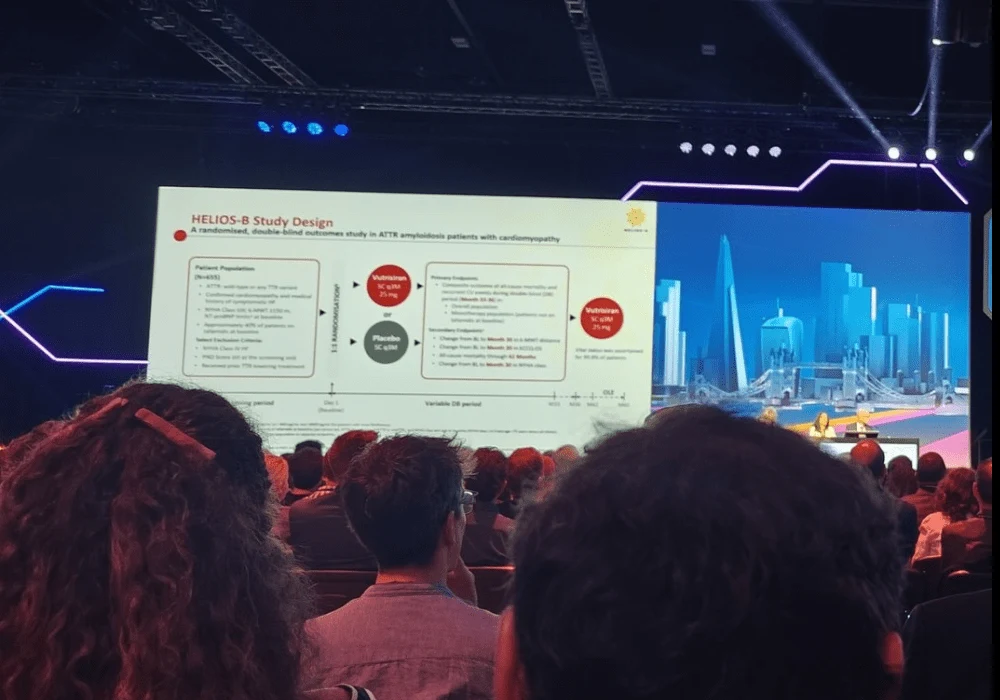
The HELIOS-B trial was a randomised, double-blind study involving patients with hereditary or wild-type ATTR-CM who showed evidence of cardiac amyloidosis via echocardiography and confirmed ATTR amyloid deposition. Patients were randomly assigned in a 1:1 ratio to receive either 25 mg of vutrisiran or placebo subcutaneously every three months for up to 36 months. Patients already receiving tafamidis, a disease stabiliser, continued their treatment.
The study's two primary endpoints were a composite of all-cause mortality and recurrent cardiovascular events at month 33, assessed in both the overall population and in those receiving vutrisiran monotherapy (not taking tafamidis at baseline). Secondary endpoints, assessed in the overall and monotherapy populations, included all-cause mortality up to 42 months, changes from baseline to 30 months in functional capacity (6-minute walk test), quality of life (Kansas City Cardiomyopathy Questionnaire Overall Summary), and New York Heart Association (NYHA) class.
A total of 655 patients were enrolled from 87 centres across 26 countries, with a median age of 76.5 years, and 92.5% were male. More than 77.6% had heart failure classified as NYHA class 2, and 40% were taking tafamidis at baseline.
The trial met its primary endpoints, with vutrisiran significantly reducing the risk of all-cause mortality and recurrent cardiovascular events by 28% in the overall population and by 33% in the monotherapy group. In a prespecified subgroup analysis, patients on background tafamidis showed a more than 20% reduction in the composite of all-cause mortality and recurrent cardiovascular events.
Over 42 months, vutrisiran reduced all-cause mortality by 36% in the overall population and by 35% in the monotherapy group compared to placebo. Other secondary endpoints related to functional capacity, health status, and quality of life also significantly improved with vutrisiran versus placebo.
Most adverse events with vutrisiran were mild or moderate, and the rates of adverse events leading to study drug discontinuation were similar between the vutrisiran (3.1%) and placebo (4.0%) groups.
These findings show that vutrisiran was highly effective and well-tolerated in this patient population and could become the new standard of care. This trial is also noteworthy as it is the first to demonstrate the benefits of gene silencers in any type of cardiomyopathy.
Source & Image Credit: ESC Congress 2024